UDC 633.521:604
V. Mishchenko1,2*, L. M. Kryvosheeva2, Yu. V. Lavrynenko3, T. Yu. Marchenko3
1Oleksandr Dovzhenko Hlukhiv National Pedagogical University, 24 Kyivska St., Hlukhiv, Sumy region, 41400, Ukraine,
*email: serhiimishchenko@ukr.net
2Institute of Bast Crops, NAAS of Ukraine, 45 Tereshchenkiv St., Hlukhiv, Sumy region, 41400, Ukraine
3Institute of ClimateOriented Agriculture, NAAS of Ukraine, 24 Maiatska doroha St., Khlibodarske, Odesa district,
Odesa region, 67667, Ukraine
Purpose. To determine the dependence of the intensity of callus formation and organogenesis of Linum usitatissimum L.
convar. elongatum in vitro on explant type and variety in order to optimize the cultivation protocol. Methods. For induction of callus formation and organogenesis, hypocotyls, cotyledons, leaves, immature embryos and anthers of flax varieties ‘Hlinum’, ‘Esman’, ‘Hladiator’, ‘Hlobus’ and ‘Charivnyi’ grown on Murashige and Skoog medium were treated with 0.05 mg/l 1naphthylacetic acid and 1.0 mg/l 6benzylaminopurine at a photoperiod of 16 h, light intensity 2500 lux, relative humidity 60–80% and air temperature 22–24 °C. Empirical data were interpreted using arithmetic mean, error of the sample mean, coefficient of variation, least significant difference and rank order. Results. The intensity of callus formation and organogenesis in the analysed varieties depended on the object of study, i.e. the genotype of the variety and the type of explant. The frequency of callus formation ranged from 9.4 (anthers of variety ‘Esman’) to 99.4% (leaf explants of variety ‘Hlinum’), the weight of callus – from 0.18 (anthers of variety ‘Esman’) to 3.18 g (anthers of variety ‘Hlobus’), the frequency of organogenesis – from 7.4 (anthers of variety ‘Esman’) to 97.3% (hypocotyls of variety ‘Hlinum’), number of shoots – from 0.6 (anthers of variety ‘Hladiator’ and immature embryos of variety ‘Hlobus’) to 4.0 (hypocotyls of variety ‘Hlinum’), height of shoots – from 0.34 (anthers of variety ‘Esman’) to 1.63 cm (anthers of variety ‘Hlobus’). Conclusions. Plants of all the varieties studied are capable of effective callus formation and organogenesis in vitro in the presence of phytohormones of exogenous origin. Certain types of explants (hypocotyls, cotyledons, leaves) respond stably to exogenous growth regulators that induce callus formation, whereas others, such as anthers, have a specific response that is largely determined by cultivar characteristics. To obtain diploid somaclones, it is optimal to use hypocotyls of varieties ‘Hlinum’ and ‘Charivnyi’, to obtain haploid regenerants – immature embryos and anthers of varieties ‘Hlobus’ and ‘Hladiator’, which ensures the highest reproduction rate of cultural plant objects.
Keywords: flax; growth medium, phytohormones; callus; somaclon, growth and development.
Serhii Mishchenko
https://orcid.org/0000000219794002
Larysa Kryvosheeva
https://orcid.org/0000000166886930
Yuriy Lavrynenko
https://orcid.org/0000000194428793
Tetiana Marchenko
https://orcid.org/0000000169943443
Introduction
Callus, suspension cell cultures and shoots regenerated from them are a selection basis for obtaining plants with increased resistance to abiotic and biotic factors of the environment: salinity, drought, diseases and pests, etc. [1–3]. Regenerated plants obtained in vitro, compared to the original material, for example, are characterized by somaclonal variability, which in the case of positive changes can be used to create new varieties. Unwanted mutant forms can be culled already at the stage of regeneration in in vitro culture.
The use of calli has become widespread in many types of crops, particularly in the case of flax (Linum usitatissimum L.) – a traditional agricultural crop with multiple economic uses, mainly grown to obtain natural fibre as a raw material for the textile industry (long-stalk flax), as well as seeds, food or technical oil (oilseed flax or long-stalk flax). In spite of significant successes in the selection of common flax, the problem of obtaining a new source of self-pollinated culture is permanent, which is why it is necessary to use in vitro technologies to solve a number of breeding tasks. Recently, the use of in vitro callus cultures of linseed and other species of the genus Linum L., obtained on the basis of stem and leaf explants, as well as cell suspension cultures for the synthesis of valuable secondary metabolites – lignans and neolignans used in medicine [4–6].
The success of using in vitro culture to obtain callus cultures and indirect organogenesis of common flax depends, first of all, on several components of the cultivation conditions: the type of nutrient medium [3, 7]; the presence of a carbohydrate source – sucrose, glucose, maltose, lactose or their combination [8, 9];
the phytohormonal composition of the growth medium, which induces intensive callus formation and organogenesis [10, 11]; the genetic characteristics of the breeding material, the type and size of explants [9, 12] and the characteristics of their preliminary preparation for inoculation [11, 13], etc.
The following combinations of phytohormones in the growth medium were described for effective induction of callus formation and organogenesis in common flax: the cytokinin 6-benzylaminopurine (BAP) alone; the cytokinin BAP in combination with the auxins 1-naphthylacetic acid (NAA) or indole-3-acetic acid (IAA) [11]. At the same time, optimal concentrations of BAP are within 1.0 mg/l ≤ BAP ≤ 1.75 mg/l; optimal concentrations of BAP in the presence of 0.05 mg/l NAA – 0.5 mg/l ≤ BAP ≤ 2.0 mg/l; optimal concentrations of NAA in the presence of 1.0 mg/l BAP – 0.025 mg/l ≤
NAA ≤ 0.150 mg/l; optimal concentrations of IAA in the presence of 1.0 mg/l BAP are
0.05 mg/l ≤ IAA ≤ 0.50 mg/l [11].
It should be noted that the protocols for in vitro cultivation of flax are quite well developed, especially with regard to exogenous growth regulators in the composition of the growth medium, as well as types of explants that induce intensive callus formation and organogenesis. However, in the role of a biotechnological object, mainly samples of the so-called oilseed flax were used, and not the long-stalk flax, which differs significantly from the first variety in terms of morphological, physiological and genetic characteristics. In connection with the creation of a series of innovative varieties [14] to optimise the cultivation protocol, the question of the dependence of the intensity of callus formation and organogenesis of Linum usitatissimum L. convar. elongatum in vitro depending on the type of explant and variety is relevant.
Materials and methods
The varieties of L. usitatissimum convar elongatum cultivated by the Institute of Bast Crops of the National Academy of Agricultural Sciences of Ukraine – ‘Hlinum’, ‘Esman’, ‘Hladiator’, ‘Hlobus’ and ‘Charivnyi’ – were used for the study. Hypocotyls, cotyledons, leaves, immature embryos and anthers were used to induce callus formation and organogenesis. The first three types of explants were taken from aseptic shoots obtained from seeds in vitro. Immature embryos and anthers were inoculated at the stage of cell vacuolation, after preliminary cold treatment (72 h at a temperature of 6 °C) and sterilisation with an aqueous solution of sodium hypochlorite (NaOCl) at a concentration of 6%, with exposure for 12.5–15 min and washing three times with sterile distilled water.
The studied explants were cultivated in culture tubes with a diameter of 20 mm on Murashige and Skoog medium with the addition of 0.05 mg/l NAA and 1.0 mg/l BAP at a photoperiod of 16 h, light intensity of 2500 lux, relative humidity of 60–80% and temperature of 22–24 °C.
On the 35th day of cultivation, the following parameters were determined: frequency of callus formation (percentage of explants on which callus was formed), mass of callus from an explant, frequency of organogenesis (percentage of calli on which shoots have formed), number of shoots formed (excluding meristematic zones and initial shoots) and height of normally developed shoots. The number of replicates is 6, the sample is at least 30 explants for each variety. The empirical data were interpreted according to the arithmetic mean, the error of the sample mean, the coefficient of variation, the least significant difference at a significance level of 0.05 and rank order.
Results and discussion
The intensity of callus formation and somatic embryogenesis depended on the object of study, namely the variety L. usitatissimum convar. elongatum and the type of explant (Table).
The highest frequency of callus formation under the influence of the investigated growth regulators (NAA and BAP) was observed on leaf and hypocotyl explants of the variety ‘Hlinum’ (99.4 and 99.3%, respectively) and leaf explants of the variety ‘Charivnyi’ (99.3%), the lowest manifestation of this characteristic – in the version using anthers of the variety ‘Esman’ (only 9.4%). The largest mass of callus on the 35th day of cultivation was formed in the variant with anthers of the ‘Hlobus’ variety (3.18 g), which can exceed other variants by more than 10 times, and the smallest mass of callus was formed on the anthers of the ‘Esman’ variety (0.18 g). There is a tendency for certain types of explants (hypocotyls, cotyledons, leaves) to respond more or less stably to exogenous growth regulators inducing callus formation, while others, such as anthers, show a specific genotypic response to this factor.
The frequency of organogenesis did not always depend on the intensity of callus formation and varied from 7.4 (anthers of the variety ‘Esman’) to 97.3% (hypocotyls of the variety ‘Hlinum’). The sign of the number of shoots formed from a group of undifferentiated callus cells was quite variable and varied significantly depending on the type of explant and the genotype of the variety. The largest number of regenerated plants was formed from callus formations on hypocotyl segments of the variety ‘Hlinum’ (4.0 pcs.).
The fewest shoots were formed from calli formed from anthers of the variety ‘Hladiator’ and immature seed embryos of the cultivar ‘Hlobus’ (0.6 each). The height of the shoots varied from 0.34 cm (anthers of the variety ‘Esman’) to 1.63 cm (anthers of the variety ‘Hlobus’), i.e. the differences reached five times.
The analysis of the coefficients of variation of the traits studied showed that their variability could be insignificant, medium and significant, while in most cases the determining influence was not so much the type of explant as the variety.
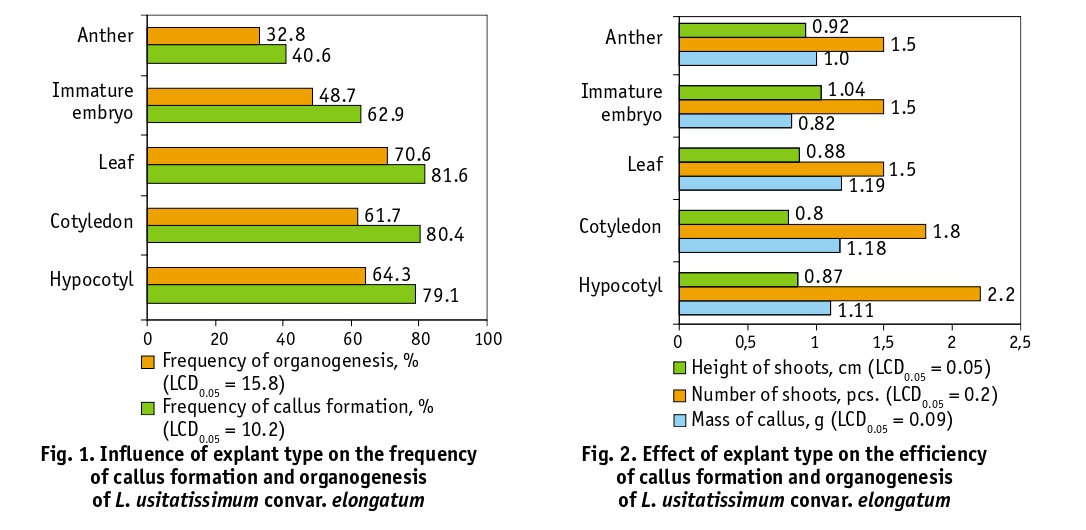
According to the average grouped data, the highest frequency of callus formation was observed when true leaves (81.6%), cotyledons (80.4%) and hypocotyls (79.1%) were used as explants, and the least capable of callus formation in vitro due to the action of NAA and BAP were anthers (40.6%). Similarly, the highest frequency of organogenesis was found on callus formations induced on leaf explants (70.6%). These indicators were quite close for the use of cotyledons, immature embryos and hypocotyls (61.7–64.3%). The lowest frequency of embryogenesis was calculated from callus formed on anthers (32.8%) (Fig. 1). The sign of the callus mass was insignificantly dependent on the type of explant, ranging from 0.82 (immature embryos) to 1.19 g (leaves). The variant with hypocotyl explants (2.2 pcs.) stood out according to the number of shoots, the least regenerants were formed on the callus of leaves, immature seed embryos and anthers (1.5 pcs. each). The sign of shoot height on the 35th day of cultivation varied from 0.80 (cotyledons) to 1.04 cm (immature embryos) (Fig. 2).

In general, to obtain somaclones of L. usitatissimum convar. elongatum in vitro from vegetative organs or their parts (provided that 0.05 mg/l NAA and 1.0 mg/l BAP are added to the Murashige and Skoog medium), it is optimal to use hypocotyl segments and to obtain regenerants from generative organs – immature embryos and anthers, which gives the highest yield of material during their further microclonal reproduction.
All studied varieties are to a large extent capable of effective callus formation and organogenesis in in vitro culture in the presence of phytohormones of exogenous origin in the environment, but at the same time there are varietal differences in this ability (genotype influence), which is manifested in the features of response to phytohormonal influence (the flow of complex physiological-biochemical processes in cells and tissues), intensity of cell dedifferentiation and differentiation, growth and development of shoots, etc. In general, the highest frequency of callus formation and organogenesis was observed in varieties ‘Hlinum’ (83.6 and 69.7%) and ‘Charivnyi’ (81.5 and 66.3%), the lowest frequency of callus formation in variety ‘Hladiator’ (51.8%), organogenesis – in variety ‘Esman’ (46.6%) (Fig. 3). At the same time, the mass of the callus ranged from 1.32 g (‘Esman’) to 2.36 g (‘Hlinum’) under the given cultivation conditions. The number of shoots was highly dependent on the genotype, with the variety ‘Hlinum’ (2.4 shoots) standing out for this characteristic. The lowest value for this characteristic was observed in the variety ‘Esman’ (1.3 shoots). The height of shoots ranged from 0.77 (‘Esman’) to 1.09 cm (variety ‘Hlinum’) (Fig. 4).

It should be noted that the use of all types of explants of the variety ‘Hlinum’ gave good results in obtaining calli and somaclones; anthers of the variety ‘Esman’ responded weakly to callus induction factors and somatic embryogenesis; the most intensive callus formation and organogenesis on anthers is characteristic of the variety ‘Hlobus’ and on immature embryos – of the variety ‘Hladiator’; the variety ‘Charivnyi’ did not have critical values of the investigated characteristics (minimum or maximum).
In general, to obtain diploids, it is optimal to use explants of the varieties ‘Hlinum’ and ‘Charivnyi’, and haploids – of the varieties ‘Hlobus’ and ‘Hladiator’, which will ensure the highest reproduction rate of the cultural plant objects of L. usitatissimum convar. elongatum.
Previously, significant differences in the ability of callus formation and organogenesis in in vitro culture due to the influence of NAA and BAP were found at the interspecies level (different Linum L. species) [7] and within varieties of the same L. usitatissimum species (elongata, intermedia and humile flax) [15]. The greatest ability to callus formation and organogenesis on hypocotyl and epicotyl explants under the same composition of the medium and cultivation conditions is characteristic of elongata flax and humile flax, the largest mass of callus from the explant, the number of regenerated shoots and their height is formed by intermedia flax, which has the largest range of variation of the studied characteristics [15]. Research on the variety ‘Lirina’ and others showed differences between the response of immature embryos (ovules) [16] and anthers [9] within a genotype of so-called oilseed flax. In order to overcome the dependence of callus formation and regeneration on varietal characteristics, a specific combination of phytohormones in the induction medium has to be modified and optimised for specific genotypes within a specific selection programme [9, 16, 17].
Conclusions
In the analysed varieties of Linum usitatissimum L. convar. elongatum ‘Hlinum’, ‘Esman’, ‘Hladiator’, ‘Hlobus’ and ‘Charivnyi’, the intensity of callus formation and embryogenesis in in vitro culture under the influence of phytohormones of exogenous origin (0.05 mg/l NAA and 1.0 mg/l BAP) depended on the object of study, i.e. the genotype of the variety and the type of explant. At the same time, the frequency of callus formation ranged from 9.4% (anthers of the variety ‘Esman’) to 99.4% (leaf explants of the variety ‘Hlinum’), the callus weight from 0.18 g (anthers of the variety ‘Esman’) to 3.18 g (anthers of the variety ‘Hlobus’), the frequency of organogenesis from 7.4% (anthers of the variety ‘Esman’) to 97.3% (hypocotyls of the variety ‘Hlinum’), number of shoots – from 0.6 (anthers of the variety ‘Hladiator’ and immature embryos of the variety ‘Hlobus’) to 4.0 (hypocotyls of the variety ‘Hlinum’), height of shoots – from 0.34 cm
(anthers of the variety ‘Esman’) to 1.63 cm (anthers of the variety ‘Hlobus’). In general, to obtain diploid somaclones, it is optimal to use hypocotyls, as well as the varieties ‘Hlinum’ and ‘Charivnyi’, to obtain haploid regenerants – immature embryos and anthers, varieties ‘Hlobus’ and ‘Hladiator’, which provides the highest reproduction rate of plant objects of the studied biological species and variety.
References
Використана література
Table
Dependence of the intensity of callus formation and organogenesis on the type of explant and the variety
of L. usitatissimum convar. elongatum
|
Type of explant |
Intensity of callus formation |
Intensity of organogenesis |
||||||||
|
Frequency of callus formation, % |
Mass of callus, g |
Frequency of organogenesis, % |
Number of shoots, pcs. |
Height of shoots, cm |
||||||
|
|
V, % |
|
V, % |
|
V, % |
|
V, % |
|
V, % |
|
|
‘Hlinum’ |
||||||||||
|
Hypocotyl |
99.3 ± 0.67 |
2.1 |
1.67 ± 0.088 |
16.7 |
97.3 ± 1.09 |
3.6 |
4.0 ± 0.24 |
18.8 |
1.13 ± 0.037 |
10.3 |
|
Cotyledon |
98.6 ± 0.93 |
3.0 |
1.48 ± 0.071 |
15.2 |
83.3 ± 2.68 |
10.2 |
2.8 ± 0.29 |
33.5 |
1.02 ± 0.025 |
7.7 |
|
Leaf |
99.4 ± 0.62 |
2.0 |
1.54 ± 0.058 |
11.9 |
82.0 ± 2.24 |
8.6 |
1.7 ± 0.03 |
6.1 |
0.92 ± 0.013 |
4.6 |
|
Immature embryos |
74.0 ± 1.20 |
5.1 |
0.78 ± 0.033 |
13.2 |
52.7 ± 4.27 |
25.6 |
1.8 ± 0.11 |
18.2 |
1.36 ± 0.056 |
13.1 |
|
Anther |
46.7 ± 1.98 |
13.4 |
0.63 ± 0.033 |
16.8 |
33.3 ± 0.99 |
9.4 |
1.5 ± 0.13 |
27.2 |
1.02 ± 0.025 |
7.7 |
|
‘Esman’ |
||||||||||
|
Hypocotyl |
97.4 ± 1.46 |
4.7 |
1.10 ± 0.037 |
10.5 |
50.4 ± 2.01 |
12.6 |
1.4 ± 0.03 |
5.8 |
0.84 ± 0.040 |
15.0 |
|
Cotyledon |
98.1 ± 1.35 |
4.4 |
1.24 ± 0.043 |
10.9 |
48.3 ± 1.10 |
7.2 |
1.3 ± 0.04 |
10.0 |
0.77 ± 0.021 |
8.8 |
|
Leaf |
98.6 ± 0.93 |
3.0 |
1.44 ± 0.040 |
8.8 |
75.2 ± 1.38 |
5.8 |
1.7 ± 0.04 |
7.6 |
0.93 ± 0.063 |
21.5 |
|
Immature embryos |
70.0 ± 3.75 |
16.9 |
0.76 ± 0.076 |
31.7 |
51.7 ± 1.93 |
11.8 |
1.4 ± 0.16 |
34.5 |
0.97 ± 0.108 |
35.1 |
|
Anther |
9.4 ± 1.47 |
49.6 |
0.18 ± 0.036 |
63.1 |
7.4 ± 0.66 |
28.4 |
0.8 ± 0.08 |
32.4 |
0.34 ± 0.065 |
60.8 |
|
‘Hladiator’ |
||||||||||
|
Hypocotyl |
52.0 ± 1.66 |
10.1 |
0.88 ± 0.146 |
52.4 |
39.3 ± 1.85 |
14.8 |
1.2 ± 0.13 |
35.1 |
0.60 ± 0.030 |
15.7 |
|
Cotyledon |
51.9 ± 1.66 |
10.1 |
1.00 ± 0.063 |
20.0 |
47.3 ± 5.84 |
39.0 |
1.3 ± 0.08 |
20.3 |
0.55 ± 0.027 |
15.4 |
|
Leaf |
52.0 ± 1.33 |
8.1 |
0.74 ± 0.056 |
24.0 |
62.0 ± 1.43 |
7.3 |
1.2 ± 0.04 |
11.4 |
0.85 ± 0.045 |
16.9 |
|
Immature embryos |
81.3 ± 2.40 |
9.3 |
1.49 ± 0.028 |
5.9 |
76.6 ± 1.12 |
4.6 |
2.5 ± 0.13 |
16.6 |
1.19 ± 0.129 |
27.5 |
|
Anther |
22.0 ± 1.74 |
25.0 |
0.43 ± 0.021 |
15.7 |
14.6 ± 1.67 |
36.0 |
0.6 ± 0.07 |
34.7 |
0.70 ± 0.033 |
15.0 |
|
‘Hlobus’ |
||||||||||
|
Hypocotyl |
48.7 ± 1.42 |
9.2 |
0.72 ± 0.073 |
31.9 |
40.0 ± 3.58 |
28.3 |
1.1 ± 0.13 |
38.5 |
0.76 ± 0.022 |
9.2 |
|
Cotyledon |
54.7 ± 3.42 |
19.8 |
0.88 ± 0.077 |
27.7 |
46.7 ± 3.72 |
25.2 |
1.8 ± 0.13 |
23.4 |
0.73 ± 0.045 |
19.4 |
|
Leaf |
58.7 ± 1.66 |
9.0 |
0.81 ± 0.048 |
18.8 |
58.0 ± 1.02 |
5.6 |
1.2 ± 0.11 |
29.1 |
0.85 ± 0.027 |
10.0 |
|
Immature embryos |
18.0 ± 1.74 |
30.5 |
0.30 ± 0.042 |
44.4 |
12.7 ± 1.84 |
45.9 |
0.6 ± 0.08 |
42.8 |
0.62 ± 0.036 |
18.3 |
|
Anther |
84.7 ± 2.23 |
8.3 |
3.18 ± 0.118 |
11.7 |
80.7 ± 1.85 |
7.2 |
3.6 ± 0.45 |
39.7 |
1.63 ± 0.065 |
12.6 |
|
‘Charivnyi’ |
||||||||||
|
Hypocotyl |
98.0 ± 1.42 |
4.6 |
1.16 ± 0.043 |
11.6 |
94.6 ± 1.33 |
4.4 |
3.1 ± 0.06 |
6.6 |
1.03 ± 0.045 |
13.8 |
|
Cotyledon |
98.7 ± 0.89 |
2.9 |
1.32 ± 0.055 |
13.3 |
82.7 ± 2.67 |
10.2 |
2.0 ± 0.15 |
24.1 |
0.94 ± 0.027 |
9.0 |
|
Leaf |
99.3 ± 0.67 |
2.1 |
1.44 ± 0.037 |
8.2 |
76.0 ± 4.24 |
17.6 |
1.7 ± 0.21 |
39.9 |
0.87 ± 0.037 |
13.3 |
|
Immature embryos |
71.4 ± 2.23 |
9.9 |
0.77 ± 0.021 |
8.8 |
50.0 ± 1.79 |
11.3 |
1.4 ± 0.14 |
30.9 |
1.06 ± 0.034 |
10.1 |
|
Anther |
40.0 ± 1.00 |
7.9 |
0.59 ± 0.031 |
16.8 |
28.0 ± 1.66 |
18.7 |
1.2 ± 0.14 |
37.0 |
0.91 ± 0.035 |
12.1 |
УДК 633.521:604
Міщенко С. В.1,2*, Кривошеєва Л. М.2, Лавриненко Ю. В.3, Марченко Т. Ю.3 Вплив типу експланта та сорту Linum usitatissimum L. convar. elongatum на інтенсивність калюсо й органогенезу в умовах in vitro. Plant Varieties Studying and Protection. 2023. Т. 19, № 3. С. 00–00. https://doi.org/10.21498/25181017.19.3.2023.287644
1 Глухівський національний педагогічний університет імені Олександра Довженка, вул. Київська, 24, м. Глухів, Сумська обл.,
41400, Україна, *email: serhiimishchenko@ukr.net
2 Інститут луб’яних культур НААН України, вул. Терещенків, 45, м. Глухів, Сумська обл., 41400, Україна
3 Інститут кліматично орієнтованого сільського господарства НААН України, вул. Маяцька дорога, 24, смт Хлібодарське,
Одеський рн, Одеська обл., 67667, Україна
Мета. Установити залежність інтенсивності калюсо й органогенезу Linum usitatissimum L. convar. elongatum в умовах in vitro від типу експланта та сорту для оптимізації протоколу культивування. Методи. Для індукції калюсо й органогенезу використовували гіпокотилі, сім’ядолі, листки, незрілі зародки та пиляки сортів льонудовгунця ‘Глінум’, ‘Есмань’, ‘Гладіатор’, ‘Глобус’ і ‘Чарівний’, які культивували на середовищі Мурасіге і Скуга, додаючи 0,05 мг/л 1нафтилоцтової кислоти та 1,0 мг/л 6бензиламінопурину, за фотоперіоду 16 год, інтенсивності освітлення 2500 лк, відносної вологості 60–80% і температури повітря 22–24 °С. Емпіричні дані інтерпретували за середнім арифметичним, похибкою вибіркової середньої, коефіцієнтом варіації, найменшою істотною різницею та ранжували. Результати. Інтенсивність калюсогенезу та органогенезу у проаналізованих сортів залежала від об’єкта дослідження. Водночас частота калюсогенезу варіювалася від 9,4 (пиляки сорту ‘Есмань’) до 99,4% (листкові експланти сорту ‘Глінум’), маса калюсу – від 0,18 (пиляки сорту ‘Есмань’) до 3,18 г (пиляки сорту ‘Глобус’), частота органогенезу – від 7,4 (пиляки сорту ‘Есмань’) до 97,3% (гіпокотилі сорту ‘Глінум’), кількість пагонів – від 0,6 (пиляки сорту ‘Гладіатор’ і незрілі зародки сорту ‘Глобус’) до 4,0 шт. (гіпокотилі сорту ‘Глінум’), висота пагонів – від 0,34 (пиляки сорту ‘Есмань’) до 1,63 см (пиляки сорту ‘Глобус’). Висновки. Рослини всіх досліджених сортів здатні до ефективного калюсо й органогенезу в культурі in vitro за наявності в середовищі фітогормонів екзогенного походження. Певні типи експлантів (гіпокотилі, сім’ядолі, листки) стабільно реагують на екзогенні регулятори росту, що індукують калюсогенез, а інші, якот пиляки, мають специфічну реакцію, що значною мірою детермінується особливостями сорту. Для отримання диплоїдних сомаклонів оптимальним є використання гіпокотилів сортів ‘Глінум’ і ‘Чарівний’, для отримання гаплоїдних регенерантів – незрілих зародків та пиляків сортів ‘Глобус’ і ‘Гладіатор’, що забезпечує найвищий коефіцієнт розмноження культуральних рослинних об’єктів.
Ключові слова: льон звичайний; живильне середовище; фітогормони; калюс; сомаклон; ріст і розвиток.
Надійшла / Received 14.08.2023
Погоджено до друку / Accepted 21.09.2023