UDC 582.688.4+582.394.744+582.711.714]:581.132.1 doi: 10.21498/2518-1017.20.1.2024.300135
B. Venediktova*, N. V. Zaimenko, N. V. Skrypchenko
M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine, 1 Sadovo-Botanichna St., Kyiv, 01014, Ukraine, *e-mail: tatianaforest3@gmail.com
The accumulation of photosynthetic pigments, biogenic elements and amino acids in the leaves of A. argute (kiwi berry) and S. chinensis (Chinese magnolia vine) during their cultivation in the same vegetative containers was studied. Different ratios of the number of plants in the containers were used, namely 50%:50%, 33%:67%, and 67%:33%. Single-species plantings were used as a control. Research demonstrated that the ratios between co-planted plants can impact the levels of photosynthetic pigments, biogenic elements, and amino acids in their leaves. Mixed planting was found to decrease the concentration of photosynthetic pigments in A. argute leaves under these conditions. The chlorophyll a content showed significant changes, decreasing by 6.7–18.7% with S. chinensis ratio in the container and by 31.3–33.8% with M. domestica ratio compared to mono-planting. Chlorophyll b also showed differences, ranging from 1.2–8.6% and 9.7–29.7%, respectively. Additionally, certain features were observed in the distribution of mineral nutrition elements in plant tissues of A. arguta. In mono-planting conditions, the leaves of plants showed an increase in magnesium, potassium, and phosphorus content. When actinidia is grown together with Chinese magnolia vine and an apple tree, especially with an apple tree, the supply of nitrogen, phosphorus, potassium, and calcium to the plants is sharply reduced. By the end of the growing season of plants, the total content of free amino acids in A. arguta leaves increased in mono-planting conditions. Actinidia plants exhibited a disturbance in phosphate metabolism in mixed plantings, as evidenced by elevated levels of arginine and histidine, as well as altered nitrogen metabolism indicated by decreased concentrations of glutamic acid in the leaves. The competitiveness analysis, using the Vanderbeng and Ennik method, showed that A. arguta plants are more compatible with S. chinensis than with M. domestica. The productivity and sustainability of crops can be significantly increased by using the method of mixed crops in agrophytocenoses. However, it is necessary to consider the bioecological features of plants and their tolerance to root secretions of other species. A. arguta and S. chinensis are forest lianas that are a unique part of forest ecosystems. They grow in multispecies groups in natural conditions, so it can be expected that they will show tolerance in mixed plantings.
Keywords: fruit vines; mixed plantings; photosynthetic pigments; biogenic element; amino acid.
Tatiana Venediktova
https://orcid.org/0000000274190703
Natalia Zaimenko
https://orcid.org/0000000323791223
Nadiia Skrypchenko
https://orcid.org/0000000212339920
Introduction
The introduction and study of new, non-traditional plant species for horticulture is of great economic importance. It is important for dietary diversity and for identifying and utilizing plants that are highly resistant to adverse environmental factors. The primary objectives of intensive gardening are to enhance the productivity and quality of agricultural products in human-exploited systems while reducing production costs [1]. The instability of the garden phytocenosis is a characteristic feature, resulting from the disruption of trophic relationships between components due to intensive use of natural resources and man-made factors [2].
Monoculture cultivation involves the long-term presence of plants in the same place, resulting in a negative feedback between plants and soil. This leads to a shift in the composition of microbial communities, which is accompanied by the depletion of nutrients in the soil, the accumulation of soil pathogens and the release of phytotoxic and autotoxic compounds due to the decomposition of plant residues. The resulting decrease in productivity and yield is known as soil fatigue or soil disease [3, 4].
Soil fatigue is a phenomenon observed in orchard-growing regions worldwide. It is characterized by changes in root structure, slow and uneven growth, and an overall decrease in biomass. In particular, soil fatigue has been found to suppress the vegetative and generative productivity of apple orchards by 50%, reduce fruit size by 10% and delay tree fruiting by 2–3 years [5].
The purpose of this work was to analyze the compatibility of A. arguta plants when grown together with S. chinensis and M. domestica. In the context of the ever-increasing problems of ecology, it is an urgent task to include the fruits of new crops in the human diet. The most important feature of most non-traditional crops is the high content of biologically active compounds in fruits and other organs. The introduction and study of new, non-traditional plant species for horticulture is of great economic importance. They are important not only because they contribute to the diversity of food rations, but also because they make it possible to identify and use plants that are highly resistant to adverse environmental factors and contain a large number of biologically active substances. The high adaptive potential of new, non-traditional crops is based on their evolutionary roots [5]. In the structure of the horticultural industry, apple is one of the most widespread fruit crops. The large number of varieties and their great potential allow apple trees to be grown in different climatic zones. Its wide distribution is associated with high winter hardiness, increased adaptability to abiotic environmental factors. abiotic environmental factors, shelf-life and good transportability of fruits.
Materials and methods
The experiments were carried out in the Department of acclimatization of fruit plants of the M. Gryshko National Botanical Garden of the NAS of Ukraine. 2-year-old plants of Actinidia arguta (Sieboldet. Zucc.). Planch. ex Miq., Schisandra chinensis (Tucrz.) Bail. and M. domestica Borkh. seedlings were chosen as objects of study. Plants were planted in March 2021 in containers with dark gray forest light loamy soil (humus content of the humate-fulvate type is 4.25%, pHKCl – 7.6, nitrogen – 2%, phosphorus – 8.2%, density – 1.2–1.4 g/cm3).The scheme of the experiment included the following options: control – single planting of A. аrguta, S. chinensis and, M. domestica plants; 1 – joint cultivation of A. аrguta and, S. chinensis at a ratio of 50%:50%; 2 – A. аrguta and S. chinensis – 67%:33%; 3 – аrguta and S. chinensis – 33%:67%; 4 – аrguta and M. domestica – 50%:50%; 5 – аrguta and M. domestica – 67%:33%; 6 – аrguta and M. domestica – 33%:67%. The experiments were carried out under controlled conditions. The temperature was maintained within 22 ± 2 ºC, and soil humidity – 60 ± 5%. Two-year-old vegetatively propagated plants were planted in the amount of 12 pieces, with a distance between plants of 5 cm). For the model experiment, 12 plants were planted in a container to maximize saturation of the soil with root exudates in order to assess the interaction between plants. The surface area of the container was 0.06 m2. The duration of the experiment was 24 weeks, due to the generally accepted technology for growing experimental plant species.
A plant receives information from another plant by changing a number of external conditions and, most importantly, by changing the allelopathic environment. This information triggers a specific response in the plant, the nature of which has been developed through evolution. This information can cause an increase in growth, an acceleration in the release of substances, a change in the direction of root growth, etc. The above-ground biomass was counted at the end of September. Samples for biochemical studies 2 leaves were taken in mid-August. The content of photosynthetic pigments in the leaves was assessed using SF-26 spectrometry. The samples were prepared in a solution of 96% alcohol for measurement. Carotenoids were measured at 440.5 nm, while chlorophyll a and b were measured at 665 nm and 649 nm respectively, with 10 repetitions. The concentration of chlorophylls was calculated using the Vermont formula, and carotenoids were calculated using the Wettstein formula [6]. The content of pigments in the extract was calculated by the following formula A = V × C / (P × 1000), where C is the concentration of pigments, mg/l; V is the volume of the extract, ml; P – the weight of plant material, g; A – pigment’ content in plant material, mg/g fresh weight. The content of biogenic elements in plant tissues was analyzed according to the method of Rinkis and Nollendorf [7] using the ICAP 6300 DUO spectrophotometer with inductively coupled plasma. Qualitative and quantitative composition of free amino acids was determined by Stein and Moore in the modification of Volochai [8] and evaluated by HPLC using Agilent 1100 chromatograph. The significance of the differences between the variants was determined using the variance method according to Fisher’s criterion and the significance level of the null hypothesis. The obtained indicators, determined with a 95% confidence interval, are trustworthy due to the high reliability of the arithmetic mean values (the calculated Student’s criterion significantly exceeds the tabular values) and the experimental error indicator is less than 5% using the Anova program.
Results and discussion
The ecophysiology of fruit crops is currently an important scientific field that allows the study of the physiological response of plants to the environment and the assessment of the functional resistance of organisms to stress conditions [9]. The influence of unfavorable factors, including long-term continuous cultivation, on plants causes certain stresses that manifest themselves at the genetic, metabolic, morphological and physiological levels [10]. Abiotic processes can directly or indirectly affect the physiological state of plant organisms, altering their metabolism, growth and development. These differences are primarily related to changes in photosynthetic activity [11].
The photosynthetic process is severely disrupted by the degradation of chloroplasts [12, 13], and the biosynthesis of chlorophylls, especially chlorophyll a, is reduced under stress conditions [14–17]. Researchers have observed significant variations in the content and ratio of photosynthetic pigments due to environmental factors. These factors include light, temperature, CO2, water, and air pollutants, and they affect the efficiency of photosynthesis in terms of ecophysiological significance [18]. Throughout the growing season of plants, the pigment complex also shows variations under the influence of environmental factors [19].
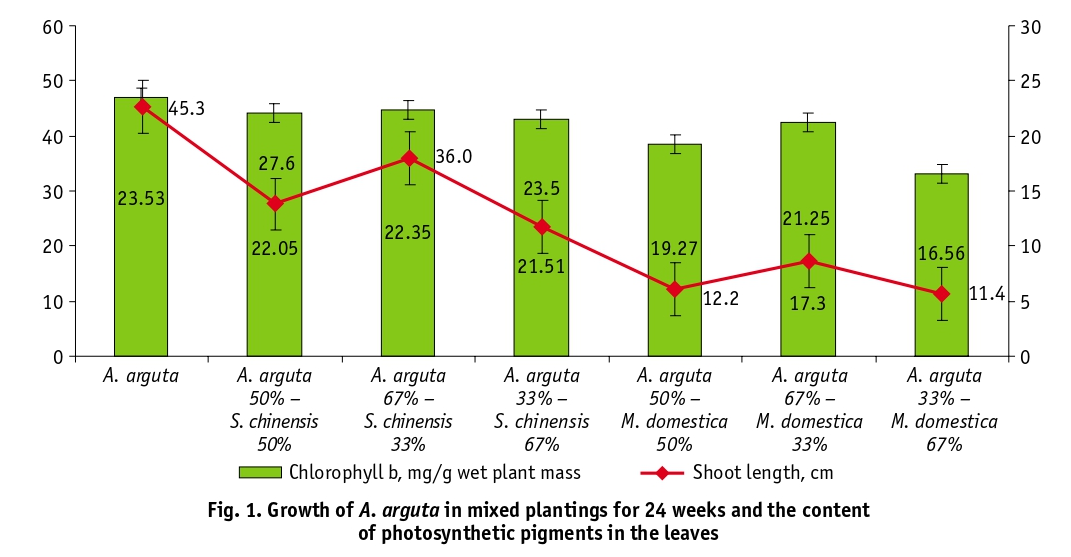
An analysis of the content of photosynthetic pigments in A. аrguta leaves showed a decrease in their concentration under conditions of mixed plantings (Table 1). The most significant changes were found in the content of chlorophyll a, whose biosynthesis decreased by 6.7–18.7% in the S. chinensis variants and by 31.3–33.8% in the M. domestica variants compared to the mono-planting. For indicators of chlorophyll b, these differences were in the range of 1.2–8.6% and 9.7–29.7%, respectively. A similar pattern was observed in the biosynthesis of carotenoids, whose content decreased on average by 1.9–61.8% compared to the control, depending on the experimental variant. It should be noted that the growth rates of аrguta plants are in agreement with the data on the concentration of chlorophyll b in the leaves (Table 1).
The study of nutrient ecology is becoming increasingly important as it is known that different soils rarely contain mineral compounds in such quantities and in such a balanced state as would be optimal for plant growth and development. Plants always compensate for the effects of stress factors through nutrition and subsequent changes in physiological adaptation to environmental conditions. Soils, in turn, are characterised by different levels of nutrients that determine the chemical composition of plants. In addition, the effect of minerals on plant growth depends on the physical, chemical and biological parameters of the soil, as well as on the external conditions and the physiological adaptation of the organisms to these changes.
Significant differences were observed in the distribution of mineral nutrients in the plant tissues of A. аrguta. In particular, the increase in the concentration of magnesium, potassium and phosphorus is observed in the leaves of plants growing under mono-planting conditions (Table 2).
Joint cultivation of A. arguta with S. chinensis and apple trees results in a violation of nutrient availability for plants, particularly magnesium, calcium, phosphorus, nitrogen, and iron. It is worth noting that the plant tissues of actinidia plants show an accumulation of manganese under these conditions, indicating soil fatigue. Furthermore, the significant presence of magnesium in leaves during mono-planting indicates that this element can easily penetrate the plant tissues of A. arguta.

Information on the content of the primary amino acids: alanine, aspartic acid, and glutamic acid, can provide important information about the relationship between primary and secondary metabolism. Aspartic acid is synthesized by direct amination, whereas the other two amino acids, alanine, and glutamic acid, are formed by reductive amination. All other amino acids are secondary, as they are formed as a result of transamination reactions of the above amino acids with the corresponding keto acids produced during metabolism, as well as by the conversion of some acids into others [20].
It was found that the total content of free amino acids in actinidia leaves increased under monoculture conditions compared to mixed planting, especially towards the end of the growing season (Table 3). This dependence can be considered as an adaptive response of the plant organism to the influence of adverse environmental factors, first of all the water supply. A disturbance in phosphate metabolism was observed in actinidia plants in mixed plantings, indicated by a higher content of arginine and histidine in the leaves of the plants, and a disturbance in nitrogen metabolism, indicated by a lower concentration of glutamic acid in the leaves of the plants.
A comparative analysis of the phytomass of A. arguta, S.chinensis, and M. domestica plants and their competitiveness according to the method of Vandenberg and Ennick [21] revealed that A. arguta plants are more competitive compared to S. chinensis vine, while M. domestica is more competitive than A. arguta (Fig. 2. а, в).
Conclusions
The study results indicate significant differences in assimilate distribution in A. arguta leaves depending on growing conditions in mono or mixed culture. Monoculture is the optimal system for growing A. arguta, as confirmed by data on the accumulation of free amino acids in actinidia leaves, indicating an increase in plant adaptive capacity. The least amount of plant inhibition was observed in mixed plantings with S. chinensis in the variant S. chinensis (33%) A. arguta (67%). The indicators of the pigment complex of plants in this variant differed slightly from those in the monoculture variant. The study revealed no significant differences in magnesium content in actinidia leaves, regardless of the plant’s growing conditions. This suggests that there is a barrier-free effect in the absorption of Mg, which is a characteristic feature of lianas.
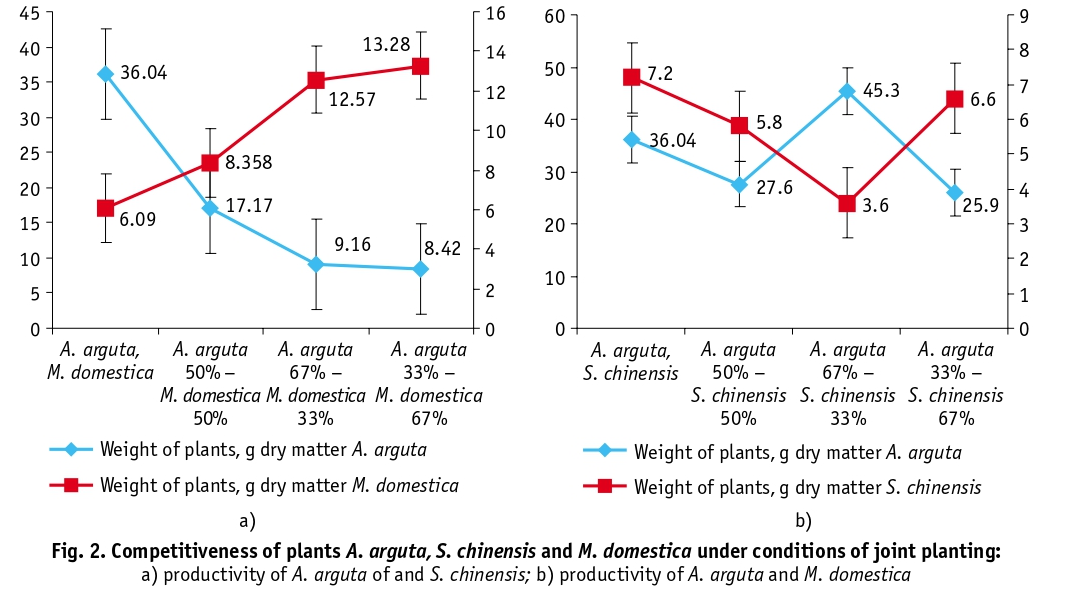
A. arguta plants are more competitive than S. chinensis, but less competitive than M. domestica. The dynamics of pigment synthesis in leaves and their total free amino acid content can be used to assess the degree of physiological stress of plants in mixed plantings. Allelopathic effect occurs as a result of accumulation of plant products in the soil and is manifested by growth retardation and reduction of plant productivity. In our experiments, when A. arguta, S. chinensis and M. domestica crops are combined, there is an accumulation of colins of soil that negatively affect the growth and development of these crops. The allelopathic potential of M. domestica is higher with A. arguta and S. chinensis.
References
Table 1
The content of photosynthetic pigments in the leaves of A. arguta under conditions of joint plantings with S. chinensis and M. domestica mg/g of wet plant mass
|
Experience variant |
a |
b |
k |
а + b |
a / b |
(a + b) / k |
|
А. аrguta |
106.99 ± 0.84 |
23.53 ± 0.75 |
40 ± 1.08 |
130.52 ± 0.31 |
4.55 ± 0.08 |
3.26 ± 0.10 |
|
А. аrguta 50% – S. chinensis 50% |
90.29 ± 1.00 |
23.25 ± 0.83 |
15.3 ± 0.39 |
113.54 ± 1.38 |
3.88 ± 0.11 |
7.42 ± 0.12 |
|
А. аrguta 67% – S. chinensis 33% |
100.25 ± 0.93 |
22.35 ± 1.13 |
39.25 ± 0.94 |
122.6 ± 0.28 |
4.49 ± 0.08 |
3.12 ± 0.07 |
|
А. аrguta 33% – S.chinensis 67% |
87.01 ± 0.43 |
21.51 ± 0.87 |
36.13 ± 0.96 |
108.52 ± 0.87 |
4.05 ± 0.16 |
3.00 ± 0.06 |
|
A. аrguta 50% – M. domestica 50% |
70.97 ± 1.24 |
19.27 ± 0.75 |
39.1 ± 0.97 |
90.24 ± 1.39 |
3.68 ± 0.1 |
2.31 ± 0.12 |
|
A. аrguta 67% – M. domestica 33% |
73.51 ± 1.43 |
21.25 ± 0.59 |
32.49 ± 1.39 |
94.76 ± 1.30 |
3.46 ± 0.14 |
2.92 ± 0.11 |
|
A. аrguta 33% – M. domestica 67% |
70.83 ± 0.87 |
16.56 ± 1.36 |
35.15 ± 0.94 |
87.39 ± 1.17 |
4.28 ± 0.1 |
2.49 ± 0.11 |
|
Maximum |
106.99 |
25.25 |
40 |
130.52 |
4.55 |
7.42 |
|
Minimum |
70.83 |
16.56 |
15.3 |
87.39 |
3.46 |
2.31 |
|
LSD0.05 |
1.37 |
1.29 |
1.31 |
1.37 |
1.15 |
0.51 |
LSD – least significant difference.
Table 2
The content of biogenic elements in the leaves of A. arguta when grown together with S. chinensis and M. domestica
|
Experience variant |
Elements, % percentage in ash |
||||||
|
macroelements |
microelements |
||||||
|
N |
P |
K |
Ca |
Mg |
Fe |
Mn |
|
|
А. аrguta |
3.3 ± 0.32 |
1.24 ± 0.04 |
3.9 ± 0.44 |
2.5 ± 0.20 |
0.69 ± 0.02 |
0.2 ± 0.036 |
0.05 ± 0.04 |
|
А. аrguta 50% – S. chinensis 50% |
2.7 ± 0.36 |
0.87 ± 0.05 |
2.8 ± 0.26 |
2.4 ± 0.21 |
0.49 ± 0.25 |
0.23 ± 0.025 |
0.06 ± 0.05 |
|
А. аrguta 67% – S. chinensis 33% |
2.9 ± 0.32 |
1.03 ± 0.04 |
3.3 ± 0.25 |
2.8 ± 0.38 |
0.51 ± 0.40 |
0.2 ± 0.04 |
0.05 ± 0.02 |
|
А. аrguta 33% – S. chinensis 67% |
2.4 ± 0.26 |
0.75 ± 0.04 |
2.5 ± 0.15 |
2.3 ± 0.15 |
0.45 ± 0.03 |
0.20 ± 0.025 |
0.06 ± 0.09 |
|
A. аrguta 50% – M. domestica 50% |
1.6 ± 0.45 |
0.51 ± 0.03 |
1.70 ± 0.21 |
0.59 ± 0.02 |
0.34 ± 0.01 |
0.16 ± 0.035 |
0.08 ± 0.05 |
|
A. аrguta 67% – M. domestica 33% |
2.0 ± 0.35 |
0.6 ± 0.041 |
2.1 ± 0.26 |
0.82 ± 0.02 |
0.38 ± 0.01 |
0.17 ± 0.049 |
0.06 ± 0.04 |
|
A. аrguta 33% – M. domestica 67% |
1.3 ± 0.40 |
0.42 ± 0.04 |
1.3 ± 0.31 |
0.47 ± 0.03 |
0.32 ± 0.04 |
0.16 ± 0.04 |
0.09 ± 0.02 |
|
LSD0,05 |
0.64 |
0.07 |
0.47 |
0.34 |
0.022 |
0.06 |
0.0198 |
Table 3
Amino acid composition of leaves of A. argute plants grown together with chinensis and M. domestica, mg/100 g of wet plant mass
|
Experience variant |
Amino acids |
||||||||||
|
Aspartic acid |
Threonine |
Serine |
Glutamic acid |
Alanine |
Histidine |
Lysine |
Arginine |
Glycine |
Proline |
Sum (∑) |
|
|
А. аrguta |
7.5 |
16.7 |
0.3 |
9.5 |
1.4 |
2.1 |
1.7 |
3.3 |
1.3 |
1.9 |
45.7 |
|
А. аrguta 50% – S. chinensis 50% |
2.9 |
10.5 |
1.2 |
4.6 |
1 |
1.4 |
0.6 |
4.7 |
0.4 |
0.3 |
27.6 |
|
А. аrguta 67% – S. chinensis 33% |
3.2 |
11.8 |
0.9 |
5.1 |
1.1 |
1.6 |
0.8 |
4.1 |
0.6 |
0.4 |
29.6 |
|
А. аrguta 33% – S. chinensis 67% |
2.6 |
10.2 |
1.5 |
4.3 |
0.8 |
1.2 |
1.1 |
5.9 |
0.7 |
0.7 |
29 |
|
A. аrguta 50% – M. domestica 50% |
2.1 |
7.7 |
1.9 |
3 |
0.4 |
0.8 |
0.4 |
7.5 |
0.1 |
0.2 |
24.1 |
|
A. аrguta 67% – M. domestica 33% |
2.3 |
8.1 |
1.7 |
3.4 |
0.6 |
1.1 |
0.6 |
6.8 |
0.4 |
0.3 |
25.3 |
|
A. аrguta 33% – M. domestica 67% |
1.8 |
7.3 |
2.4 |
2.6 |
0.3 |
0.7 |
0.8 |
8.6 |
0.4 |
0.2 |
25.1 |
|
LSD0,05 |
1.69 |
1.8 |
1.44 |
1.63 |
1.2 |
1.14 |
1.3 |
1.17 |
1.02 |
0.7 |
2.08 |
УДК 582.688.4+582.394.744+582.711.714]:581.132.1
Венедиктова Т. Б., Заіменко Н. В., Скрипченко Н. В. Сумісність Actinidia arguta з рослинами Schisandra chinensis і Malus domestica у змішаних насадженнях. Plant Varieties Studying and Protection. Т. 20, № 1. С. 39–44. https://doi.org/10.21498/25181017.20.1.2024.300135
Національний ботанічний сад імені М. М. Гришка НАН України, вул. СадовоБотанічна, 1, м. Київ, 01014, Україна,
*email: tatianaforest3@gmail.com
Досліджено особливості накопичення фотосинтетичних пігментів, біогенних елементів та амінокислот у листі A. argute (ківі) та S. chinensis (лимонника китайського) під час їх культивування в одних і тих самих вегетаційних контейнерах. Співвідношення кількості рослин у контейнерах були різними: 50:50%, 33:67% і 67:33%. Як контроль використовували одновидові насадження. Встановлено, що співвідношення між спільно висадженими рослинами можуть впливати на рівні фотосинтетичних пігментів, біогенних елементів і амінокислот в їхньому листі. Водночас виявлено зменшення концентрації фотосинтетичних пігментів у листках A. argute за умови змішаних насаджень. Найсуттєвіше знизився вміст хлорофілу
а – на 6,7–18,7% у варіантах з S. chinensis у контейнері та на 31,3–33,8% у варіантах з M. domestica, як порівняти з мононасадженнями. Відмінності для хлорофілу b були в межах 1,2–8,6% і 9,7–29,7% відповідно. Крім того, спостерігали певні особливості розподілу елементів мінерального живлення в рослинних тканинах A. arguta. В умовах мононасаджень у листках збільшувався вміст магнію, калію та фосфору. За спільного вирощування актинідії з лимонником китайським і особливо з яблунею різко зменшувалося надходження до рослин азоту, фосфору, калію та кальцію. В мононасадженнях у листках A. arguta до кінця вегетації підвищувався загальний вміст вільних амінокислот. У змішаних насадженнях рослини актинідії продемонстрували порушення метаболізму фосфатів, про що свідчать підвищені рівні аргініну та гістидину, а також зміну метаболізму азоту, що видно зі зниження концентрації глутамінової кислоти у листі. За результатами проведеного методом Вандербенга та Енніка аналізу конкурентоспроможності можна зробити висновок, що рослини A. arguta більш сумісні з S. chinensis, ніж з M. domestica. Продуктивність і стійкість насаджень можна істотно підвищити, використовуючи метод змішаних посівів в агрофітоценозах. Проте необхідно зважати на біоекологічні особливості рослин та їхню толерантність до кореневих виділень інших видів. A. arguta та S. chinensis – лісові ліани, які є унікальною частиною лісових екосистем; у природних умовах ростуть багатовидовими угрупованнями, тому є толерантними в змішаних насадженнях.
Ключові слова: плодова лоза; змішані насадження; фотосинтезуючі пігменти; біогенний елемент; амінокислота.
Надійшла / Received 03.03.2024
Погоджено до друку / Accepted 21.03.2024