UDC 546.62:581.44./45:582.683.2:502.127 https://doi.org/10.21498/25181017.20.2.2024.304093
M. Gaponenko1*, A. M. Gnatiuk2, A. V. Salnikova1, D. B. Rahmetov2
1National University of Life and Environmental Sciences of Ukraine, 13 Horikhuvatskyi shliakh St., 03041, Kyiv, Ukraine, *email: andrreygaponenko@gmail.com
2M. M. Gryshko National Botanical Garden, National Academy of Sciences of Ukraine, 1 Sadovo-Botanichna St., Kyiv, 01014, Ukraine
Purpose. To determine the level of aluminum (Al) accumulation in plants of the Brassicaceae family, which are used in agriculture as green manure and are promising plants for soil phytoremediation. The following crops were the subject of the study: oilseed radish, variety ‘Kyianochka’ (Raphanus sativus L. var oleiformis Pars. ‘Kyianochka’), white mustard, variety ‘Soniachna’ (Sinapis alba L. ‘Soniachna’), winter rapeseed, variety ‘Horlytsia’ (Brassica napus L. ‘Horlytsia’), Sarepta mustard, variety ‘Zolotava’ (Brassica juncea (L.) Czern. ‘Zolotava’), winter turnip rape, variety ‘Oriana’ (Brassica campestris var. oleifera f. biennis D.C. ‘Oriana’), tyfon, variety ‘Fitopal’ (Brassica campestris var. oleifera f. biennis DC. ´ Brassica rapa L. ‘Fitopal’). Methods. The research was carried out in the M. M. Gryshko National Botanical Garden of the National Academy of Sciences of Ukraine (Kyiv). Plants grown as green manure crops for 56 days on grey forest degraded sandy loam soil, pH 6.5–7.0, were analysed. Aluminum content was determined using an inductively coupled plasma optical emission spectrometer (ICPOES) ICAP 6300 Duo. The possibility of metal accumulation in plant tissues was assessed using the bioconcentration factor (BF). Results. Measurements showed the following content of Al in plant tissues (in abs. dry matter): oilseed radish, variety ‘Kyianochka’ – 2035.9 mg/kg, white mustard, variety ‘Soniachna’ – 687.5 mg/kg, winter rapeseed, variety ‘Horlytsia’ – 388.6 mg/kg, Sarepta mustard, variety ‘Zolotava’ – 1238.5 mg/kg, winter turnip rape, variety ‘Oriana’ – 1105.2 mg/kg, tyfon, variety ‘Fitopal’ – 854.4 mg/kg. Conclusions. Under growing conditions, the aluminum content in plants did not exceed 0.2%. All the samples studied have a BF < 1 and are not hyperaccumulators of the element according to this criterion. However, three of the investigated samples (‘Zolotava’ Sarepta mustard, ‘Oriana’ winter turnip rape and ‘Kyianochka’ oilseed radish) have an aluminum content in the aboveground dry matter of more than 1000 mg/kg, indicating a significant accumulation of this element. For the purposes of phytoextraction of aluminum, the most suitable of the plants studied is oilseed radish ‘Kyianochka’ (BF ≈ 0.4).
Keywords: phytoremediation; phytoextraction; accumulation of metals; Brassica; Sinapis; Raphanus; chemical composition.
Andrii Gaponenko
https://orcid.org/0000-0003-4215-2216
Alla Gnatiuk
https://orcid.org/0000-0001-5001-971X
Anna Salnikova
https://orcid.org/0000-0001-6706-2140
Dzhamal Rakhmetov
https://orcid.org/0000-0001-7260-3263
Introduction
The use of green manure is one of the most effective methods of returning organic matter to the soil, which has a positive effect on overall fertility, and is also a method of natural soil restoration and reclamation. The cultivation of phytoremediation plants has a positive effect on the biological activity and structure of contaminated and degraded soils [1]. The use of plants from the Brassicaceae family, such as Sarepta mustard [Brassica juncea (L.) Czern.], white mustard (Sinapis alba L.), winter rapeseed (Brassica napus L.), tyfon (Brassica campestris var. oleifera f. biennis DC. × Brassica rapa L.), oilseed radish (Raphanus sativus L. var. oleiformis Pars.) are effective for this purpose [2–4].
Crop diversification, implementation and integration of different methods of agricultural production on degraded and disturbed soils include the cultivation of plants for bioremediation of chemical elements [1]. The Brassicaceae family contains many metal accumulating species [5]. Members of the genus mustard (Sinapis L.) are recognized as the most valuable phytomeliorants, accumulating cadmium, lead, zinc and synthesizing phytic acid by physical adsorption on heavy-metal contaminated soils [1].
Aluminum (Al) is known to be the third most abundant metal in the Earth’s crust [6] and has the most versatile applications of all metals. In particular, it is used in the engineering, construction, household, cosmetics, food, chemical and medical industries [7]. Most aluminum compounds enter the environment through waste. According to the U. S. Aluminum Association, about a quarter of the world’s aluminum production is used for packaging (containers, cans, lids, caps, household foil, foil-based laminates, etc.) [8].
Aluminum compounds in soil solution play a crucial role in soil chemical processes and soil fertility [9]. Aluminum can form highly insoluble compounds with soil anions such as phosphates and sulphates. The concentration and activity of Al3+ in soil solutions depend on the processes by which aluminum is partitioned between the solid and liquid phases of the soil, as well as on many reactions in the soil solution [10].
In the pH range (4.5–7.0) typical of Ukrainian Polissia soils, different forms of aluminum are present in solution: Al3+ or Al(H20)6, AlOH2+, Al(OH)2, Al(OH)3, Al(OH)4 [9]. Their ratio depends on the pH. Organic matter can retain aluminum through a process called specific adsorption or complexation, reducing its availability to plants. The formation of aluminum humic and fulvic complexes in soils is largely determined by the distribution of natural organic matter in the soil profile [9]. However, the chemical process of Al interaction in soils is extremely complex [11].
Al content is considered an important limiting factor for plant growth and productivity on acidic soils. At pH levels below 5.5, Al becomes soluble and changes from its hydroxide form to toxic forms. This can cause a negative response in Al-sensitive plants, including inhibition of root growth and damage to their tissues, leading to further nutrient and water deficits due to impaired uptake [11]. The phytotoxic effect of aluminum is enhanced by the combined action of aluminum and iron ions, aluminum and manganese, and by soil deficiencies of phosphorus, calcium, magnesium and molybdenum [12]. There is evidence that the use of aluminum can reduce H+ toxicity and increase P availability for many agricultural crops [13].
At the same time, there is information that aluminum can be beneficial to plants, stimulating growth and alleviating biotic and abiotic stresses [6, 11]. It is involved in the formation of stable soluble complexes with natural organic acids, which has an effect on the supply of biogenic elements to plants [9].
Some plants are able to accumulate quite high levels of Al. These plants include the highland bent (Agrostis castellana Boiss. & Reut.), barley (Hordeum vulgare L.), species of the genus goldenrods (Solidago L.), horse bean (Vicia faba L.) and species of the genus hortensia (Hydrangea Gronov. ex L.) [11, 14–16]. Representatives of the family Symplocaceae Jacq. contain large amounts of Al in the tissues of the aerial parts of the plants. The Al concentration in the leaves of Asiatic sweetleaf (Symplocos paniculata Miq.), expressed as dry weight, was 4107 (± 1474) mg/kg and 4290 (± 1025) mg/kg for seedlings and saplings, respectively [17]. Plants such as camellias (Camellia L.), the jolcham oak (Quercus serrata Murray), the Arabica coffee (Coffea arabica L.), Vochysia tucanorum (Vochysia tucanorum Mart.) and Malabar melastome (Melastoma malabathricum L.) are also considered to be hyperaccumulators of Al [11]. In particular, the aluminum content in plants of Malabar melastoma (M. malabathricum) was as follows: in young leaves – 8.0 mg/g, mature leaves – 9.2 mg/g, old leaves – 14.4 mg/g and roots – 10.1 mg/g [18].
The use of certain mineral or biofertilisers has been shown to reduce aluminum toxicity [11]. Mycorrhizae have been shown to help plants tolerate aluminum toxicity by improving nutrient uptake and providing a physical barrier. Bacteria also contribute to plant resistance to aluminum toxicity, possibly through mechanisms such as altering soil pH [19]. Application of calcium, magnesium, sulphur, phosphorus, boron and silicon has been shown to reduce aluminum toxicity to plants [19].
The study of green manure plants in terms of their accumulation of certain chemical elements, particularly aluminum, and their possible use in soil phytoremediation is relevant. Therefore, the aim of our study was to determine the level of Al accumulation in plants of some members of the Brassicaceae family, which are used in agriculture as green manure and are promising plants for soil phytoremediation.
Materials and methods
The study was conducted at the experimental plots of the M. M. Gryshko National Botanical Garden of the National Academy of Sciences of Ukraine in Kyiv (hereinafter NBG) in 2023. The subject of the study were varieties: oilseed radish, variety ‘Kyianochka’ (Raphanus sativus L. var oleiformis Pars. ‘Kyianochka’), white mustard, variety ‘Soniachna’ (Sinapis alba L. ‘Soniachna’), winter rapeseed, variety ‘Horlytsia’ (Brassica napus L. ‘Horlytsia’), Sarepta mustard, variety ‘Zolotava’ (Brassica juncea (L.) Czern. ‘Zolotava’), winter turnip rape, variety ‘Oriana’ (Brassica campestris var. oleifera f. biennis D.C. ‘Oriana’), tyfon, variety ‘Fitopal’ (Brassica campestris var. oleifera f. biennis DC. × Brassica rapa L. ‘Fitopal’) of the NBG breeding. They were grown from seed as green manure after row crops in the experimental field. The soil in this area is grey forest degraded due to long-term agricultural use, low in humus, sandy loam, pH 6.5–7.0. The whole territory of the experimental field was divided into equal plots of 25 m2 in 4 replications. Plants were grown for 56 days (8 weeks from 24 September to 14 November) before the onset of frost. At the time of the study, all plants were in the virgin ontogenetic state. Plants (their above-ground part) for analysis were randomly selected by transect from each plot separately and a generalized sample was formed for each sample (variety). Samples of topsoil to form a generalized sample were taken from a control plot free of experimental plants, which was left ‘fallow’ during the growing season.
The aluminum content was determined by measuring the concentration of the chemical element using an inductively coupled plasma optical emission spectrometer (ICP-OES) ICAP 6300 Duo (Thermo Fisher Scientific Corporation, USA) at the Spectrometric Centre for Elemental Analysis at the NBG of the National Academy of Sciences of Ukraine. Instrument calibration and elemental content measurements were performed in accordance with the established methods of operation of the optical emission spectrometer and the technical documentation of the instrument. The methodology is based on wet digestion of organic matter. All reagents were of recognized analytical grade. From the generalized sample of each selected specimen prepared for chemical analysis, a weight of (500 ± 50) mg was taken and acid soluble forms of metals were extracted. The samples were decomposed by the wet ashing method using 30% nitric acid (HNO3, grade OSCH) in a special high pressure system in a microwave oven MWS-2 (Berghoff, Germany). After cooling, the resulting mineralizate was mixed with bidistilled water (obtained in a borosilicate glass apparatus) and analyzed. The soil samples were analyzed according to the traditional method [20]. The limits of the relative uncertainty of the measurement results (U) of the mass fractions of the chemical element in the samples did not exceed 20% (at k = 2, P = 0.95). The intra-laboratory control of the accuracy of the measurement results was carried out using a certified standard moss sample M2 (Moss Reference Material M2, Pleurozium schreberi – The Finnish Forest Research Institute), using the compatibility criterion. The aluminum content (mg/kg) was measured in the raw samples and then converted to the absolute dry weight of the analyzed material.
The possibility of metal accumulation in plant tissues was assessed using the BF – bioconcentration factor [21], defined as
BF = Kp / Ks,
where Kp is the metal concentration in the plant and Ks is the metal concentration in the soil.
The BF is important for screening hyperaccumulators for phytoremediation purposes when considering the potential of a particular candidate species for phytoremediation. Plant species with a BF greater than 1 can be successfully used for phytoremediation. Hyperaccumulators have a BF greater than 1, sometimes reaching 50–100 [22]. Microsoft Excel 2016 was used to graph the research results and calculate statistical indicators.
Results and discussion
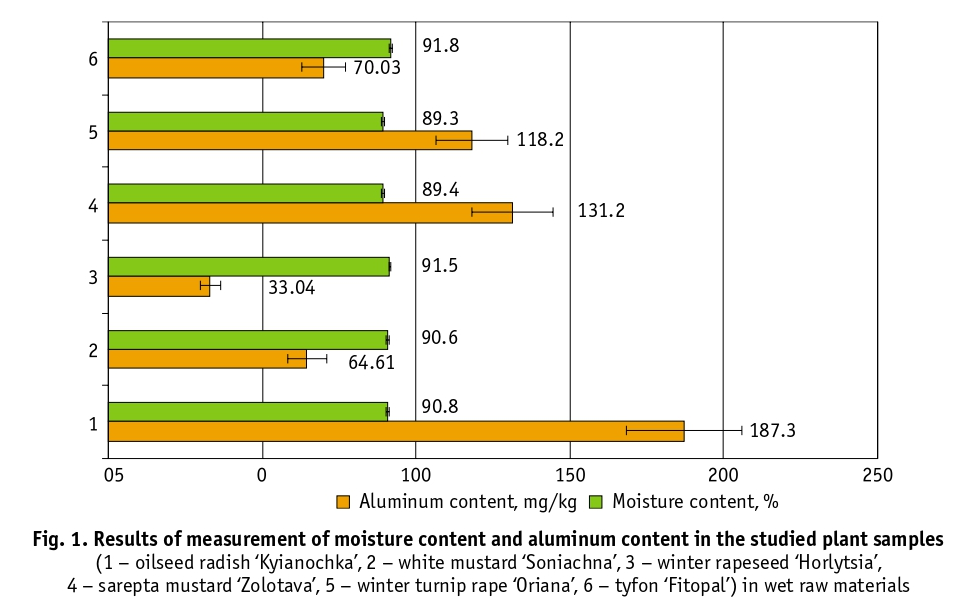
The samples of plant material contained 89–91% moisture and the aluminum content ranged from 33.04 to 187.3 mg/kg (Fig. 1). These data can be useful because the plants studied are multifunctional and their green mass can be used for animal feed [2].
The chemical composition of plants reflects to some extent the elemental composition of soils. The accumulation of an element by plants is therefore primarily determined by its presence in the soil and the characteristics of the plants themselves. In their vital activities, plants only come into contact with available forms of aluminum, the amount of which is closely related to the buffering capacity of soils. An accumulation of aluminum of 1000 mg/kg or more in absolutely dry leaf tissue is considered to be a sufficient criterion for determining aluminum hyperaccumulation [23]. According to literature data, barley (Hordeum vulgare) in particular accumulates about 1000 mg/kg of aluminum and this plant is classified as a hyperaccumulator of this element [14, 16].

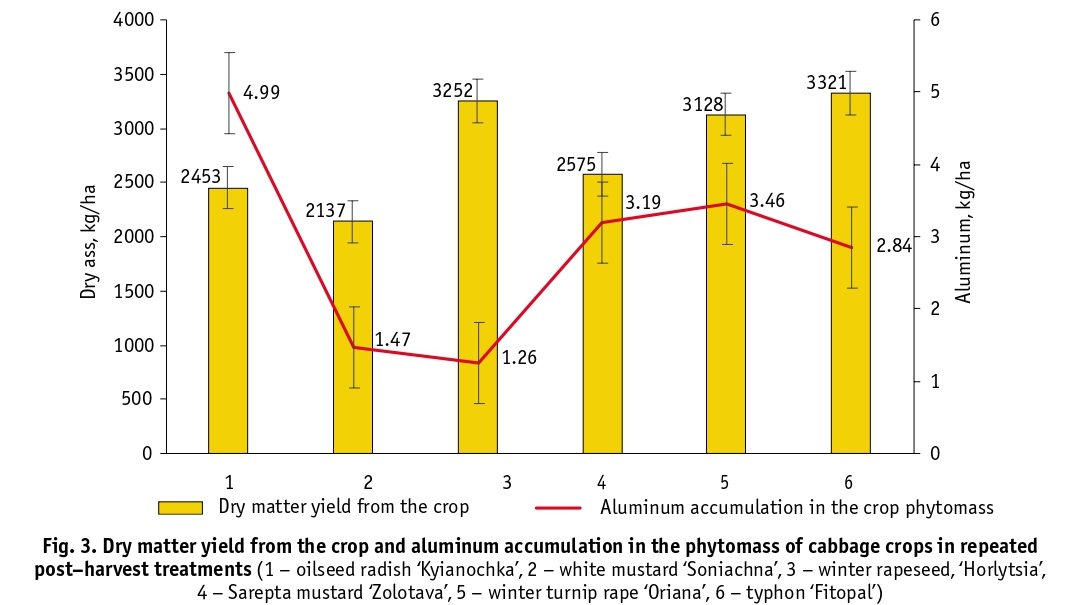
The typical range of aluminum content in soils is variable and ranges from 1 to 30% [24]. Soil analysis at the experimental site showed that the aluminum content (5810 mg/kg) in the topsoil was not high. Soils in other areas of the NBG contain aluminum in the range of 6000 to 17 000 mg/kg [25]. Given the low aluminum content and the near-neutral soil pH, the difference in the accumulation of this element by different plant species under the same growing conditions was, in our opinion, significant. Thus, the largest amount of aluminum was accumulated by oilseed radish, variety ‘Kyianochka’ – 2036 mg/kg (BF about 0.35), winter rapeseed, variety ‘Horlytsia’ showed the lowest ability to accumulate this element – 380 mg/kg (BF about 0.07) (Fig. 2). Comparison of the data on accumulation of dry matter and aluminum in phytomass by the studied crops (Fig. 3) showed a weak correlation between these indicators (Pearson’s coefficient, R = – 0.13), which indicates the species-specificity of aluminum accumulation.
All the analysed plants used aluminum. Its content in the samples ranges from 300 to almost 2500 mg/kg. All the samples studied have a BF of less than 1 and are not hyperaccumulators of the element according to this criterion. However, three of the tested samples have an aluminum content in dry weight of more than 1000 mg/kg, which indicates a significant accumulation of this element in the aerial parts of the plants. The most successful accumulator among the plants studied is oilseed radish, variety ‘Kyianochka’, which accumulated almost twice as much aluminum as other plants.
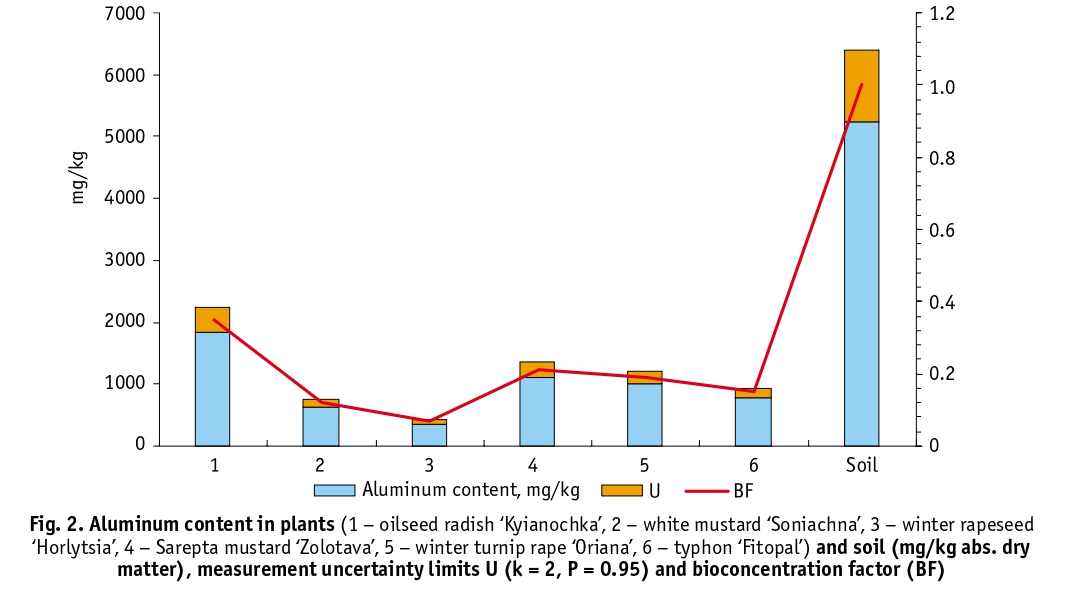
The aluminum content in the tissues of this taxon is almost 40% of the soil content. Laboratory studies carried out by Ya. H. Tsytsiura [4] on the leaf mass of plants sown after the harvest of oilseed radish showed that plants accumulate on average 43–62 kg/ha of nitrogen, 18–22 kg/ha of phosphorus and 68–80 kg/ha of potassium, which confirms the high ability of this crop to accumulate the main mineral compounds. In addition to oilseed radish, Sarepta mustard ‘Zolotava’ and winter turnip rape ‘Oriana’ also exceeded the 1000 mg/kg threshold. Their aluminum content is almost the same (within the measurement uncertainty). Winter rapeseed, variety ‘Horlytsia’, consumed and accumulated the least aluminum (388.6 mg/kg on average).
Conclusions
All the Brassicaceae species studied were found to be capable of accumulating aluminum. Under the conditions of their cultivation as green manure after harvesting in the pre-generative period of plant ontogeny, the aluminum content (expressed as abs. dry matter) in the above-ground part did not exceed 0.2%. The aluminum accumulation limit of 1000 mg/kg was exceeded by Sarepta mustard of the variety ‘Zolotava’, winter rape of the variety ‘Oriana’ and oilseed radish of the variety ‘Kyianochka’. Oilseed radish of the variety ‘Kyianochka’ was identified as the most suitable among the plants studied for the purpose of aluminum phytoextraction (BF ≈ 0.4).
References
УДК 546.62:581.44./45:582.683.2:502.127
Гапоненко А. М.1*, Гнатюк А. М.2, Cальнікова А. В.1, Рахметов Д. Б.2 Накопичення алюмінію в наземних частинах рослинсидератів з родини Вrassicaceae. Plant Varieties Studying and Protection. 2024. Т. 20, № 2. С. 00–00. https://doi.org/10.21498/25181017.20.1.2024.304093
1Національний університет біоресурсів і природокористування України, вул. Горіхуватський шлях, 13, корп. 4, м. Київ, 03041, Україна, *email: andrreygaponenko@gmail.com
2Національний ботанічний сад імені М. М. Гришка НАН України, вул. СадовоБотанічна, 1, м. Київ, 01014, Україна
Мета. Встановити рівень накопичення алюмінію (Аl) у рослинах представників родини Brassicaceae, використовуваних як сидерати у сільському господарстві та перспективних для фіторемедіації ґрунтів. Предметом дослідження були такі культури, як редька олійна [cорт ‘Кияночка’ (Raphanus sativus L. var oleiformis Pars. ‘Kyianochka’)], гірчиця біла [сортозразок ‘Сонячна’ (Sinapis alba L. ‘Soniachna’)], ріпак озимий [сортозразок ‘Горлиця’ (Brassica napus L. ‘Horlytsia’)], гірчиця сарептська [сортозразок ‘Золотава’ (Brassica juncea (L.) Czern. ‘Zolotava’)], суріпиця озима [сорт ‘Оріана’ (Brassica campestris var. oleifera f. biennis D.C. ‘Oriana’)], тифон [сорт ‘Фітопал’ (Brassica campestris var. oleifera f. biennis DC. × Brassica rapa L. ‘Fitopal’)]. Методи. Дослідження проводили в Національному ботанічному саду імені М. М. Гришка НАН України (м. Київ). Рослини, що вирощували як сидерати, аналізували протягом 56 днів на сірому лісовому деградованому супіщаному малогумусному ґрунті, pH 6,5–7,0. Вміст алюмінію визначали, використовуючи оптичний емісійний спектрометр з індуктивно зв’язаною плазмою (ІCPОЕS) ІCAP 6300 Duo. Можливість накопичення металу в тканинах рослин оцінювали за допомогою біоконцентраційного фактора (ВF). Результати. Здійснивши вимірювання, встановили такий вміст Аl у тканинах рослин (у перерахунку на абсолютно суху речовину): редька олійна (сорт ‘Кияночка’) – 2035,9 мг/кг; гірчиця біла (сортозразок ‘Сонячна’) – 687,5; ріпак озимий (сортозразок ‘Горлиця’) – 388,6; гірчиця сарептська (сортозразок ‘Золотава’) – 1238,5; суріпиця озима (сорт ‘Оріана’) – 1105,2; тифон (сорт ‘Фітопал’) – 854,4 мг/кг. Висновки. Вміст алюмінію в рослинах за наявних умов вирощування не перевищував 0,2%. Всі досліджені зразки мали показник BF < 1 та не були гіпернакопичувачами елемента за цим критерієм. Однак сортозразок гірчиці сарептської ‘Золотава’, сорт суріпиці озимої ‘Оріана’ та сорт редьки олійної ‘Кияночка’ продемонстрували значне накопичення алюмінію в наземній сухій масі – понад 1000 мг/кг. Найпридатнішою для фітоекстракції алюмінію серед досліджуваних рослин виявилася редька олійна ‘Кияночка’ (BF ≈ 0,4).
Ключові слова: фіторемедіація; фітоекстракція; накопичення металів; Brassica; Sinapis; Raphanus; хімічний склад.
Надійшла / Received 19.03.2024
Погоджено до друку / Accepted 28.05.2024