UDC 577.1 doi: 10.21498/25181017.20.2.2024.304094
O. Molodchenkova*, S. V. Koblai, P. S. Tykhonov, L. Ya. Bezkrovna, V. Ryshchakova, Yu. A. Levitsky, I. A. Untilova
Plant Breeding and Genetics Institute – National Center of Seed and Cultivar Investigation, 3 Ovidiopolska doroha St., Odesa, 65036, Ukraine, *email: olgamolod@ukr.net
Purpose. To study the biochemical parameters characterising seed quality in pea varieties of different morphotypes for the selection of genotypes with improved nutritional properties. Methods. Seeds of 37 different morphotypes [leafy, leafless, heterophillous (chameleon)] of domestic and foreign pea varieties were studied. Standard and laboratory developed methods of biochemical analysis of plants (Kjeldahl method, spectrophotometric methods, electrophoresis) were used. The statistical analysis of the research results was carried out using the software LibreOffice Calc (GNU Lesser General Public License v3) and the image analysis software Imagel. Results. The presence of varietal differences in the biochemical parameters studied related to seed quality (protein content, flavonoids, lipoxygenase activity, trypsin inhibitor, lectins), the content of the main fractions of the protein complex (legumin and vicilin) and their ratio in seeds of different morphotypes was established. The electrophoretic and amino acid analyses revealed varietal differences (in the relative content of certain protein components in the electropherogram, the presence/absence of some components in the electrophoretic spectra of vicilin and legumin, and their amino acid composition) that affect the nutritional value of pea seeds. Conclusions. The application of the biochemical criteria studied makes it possible to select varieties of food peas with specific technological parameters.
Keywords: pea; seed quality; protein; vicilin; legume; flavonoids; antinutritional factors.
Olga Molodchenkova
https://orcid.org/0000-0003-2511-0866
Svitlana Koblai
https://orcid.org/0000-0002-4509-2717
Pavlo Tykhonov
https://orcid.org/0000-0001-8738-7946
Lidiya Bezkrovna
https://orcid.org/0000-0003-2227-1541
Olga Ryshchakova
https://orcid.org/0000-0003-0621-6171
Yuri Levitsky
https://orcid.org/0000-0003-1203-8498
Iryna Untilova
https://orcid.org/0009-0001-7072-5141
Introduction
Peas (Pisum sativum L.) have long been cultivated and are becoming increasingly important for agriculture worldwide due to their valuable food and fodder properties, high yields, and the possibility of using them as the best predecessor for cereals [1]. Today, pea culture is represented by various morphotypes: leafy, leafless, heterophillous and lupinoid forms [2]. Pea seeds contain 20–35% protein, starch, sugars, fat, vitamins (A, B2, B6, C, PP, K, E), carotene, minerals (potassium, calcium, manganese, iron, phosphorus salts). Pea proteins are characterised by good solubility, digestibility and high biological value. They contain all the essential amino acids necessary for the normal functioning of the body. Therefore, growing this crop plays an important role in solving both the problem of vegetable protein deficiency and providing valuable food, dietary and medicinal products [3, 4].
It was found that the most promising proteins for the production of foods from pulses are globulin fractions with sedimentation constants of 7S and 11S, and their content and ratio in total protein determine its quality, since they are unbalanced in amino acid composition [5]. Pea storage proteins consist mainly of three globulins – legumin, vicillin and convicillin. Pea legumin is a hetero-oligomer with a molecular weight of 330–410 kDa. It consists of six polypeptides with a molecular weight of ~ 60 kDa, containing an acidic α (38–40 kDa) and a basic β (19–20 kDa) subunit (α + β) linked by disulfide bonds. Pea vicillin is a protein with a molecular weight of 150–170 kDa and a trimeric structure consisting of 3 subunits (α + β + γ). Pea convicillin is a protein with a molecular weight of about 290 kDa and a trimeric structure. It is known that legume and vicillin are glycolated and contain covalently bound carbohydrates: the former – glucose, mannose and glucosamine, and vicillin fractions, mainly mannose [6, 7]. Pulses contain a significant amount of polyphenolic compounds, particularly flavonoids, which are natural antioxidants with a wide range of biological activities [8, 9].
The nutritional properties of legume seeds are associated with the presence of substances such as trypsin inhibitors, lectins, lipoxygenase, which negatively affect the nutritional and feed value of seeds. For example, because trypsin inhibitors block the active centers of gastrointestinal enzymes, the digestibility of dietary proteins is reduced, which increases the deficiency of essential amino acids and causes pancreatic hypertrophy. Pea trypsin inhibitors are similar in structure to the well-characterized Baumann-Birk inhibitors found in soybeans. These proteins contain two active sites that mainly inhibit proteolytic enzymes (trypsin and chymotrypsin) [10]. Heat treatment has been shown to reduce the activity of protease inhibitors in pea seeds by up to 86%, but this affects the nutritional value of the seeds by reducing the content of lysine, methionine and cysteine [11]. Lectins are proteins with specific biological properties that can reversibly and selectively bind carbohydrates without causing their chemical transformation. These proteins can cause digestive problems and contribute to inflammatory diseases, such as rheumatoid arthritis and type 1 diabetes, by binding to cells for long periods of time and triggering an autoimmune response [12]. Lipoxygenase (EC number 1.13.11.12) is an iron-containing dioxygenase that catalyses the hydroperoxidation of linoleic acid and other polyunsaturated fatty acids and their esters, as well as glycerides with cis,cis-1,4-pentadiene structures. The products formed during the enzymatic reaction are very important for food quality. Hydroperoxide derivatives and their degradation products can react with proteins, peptides and amino acids, resulting in off-flavours and reduced nutritional value. The action of lipoxygenase also generates free radicals that can react with and destroy chlorophylls, carotenoids, ascorbic acid, phenols and alpha-tocopherol [13].
Pea breeding is carried out in many directions: grain, forage, food, vegetable and technical [14]. The expediency and effectiveness of breeding work to improve the habitus of pea plants, as well as the possibility of their further use as a source material for improving seed quality and for food purposes, have been established [15, 16]. The development of the system of production, processing of leguminous crops and use of legume products for food purposes has significantly increased the requirements for seed quality and brought to the fore the problem of creating new high quality varieties for food use. In this context, the study of the biochemical composition of seeds of new pea varieties and the inclusion of genotypes with the required biochemical composition in the breeding process to create varieties with improved nutritional properties is an urgent problem and of considerable theoretical and practical importance. Carrying out such research will enable breeders to direct their breeding efforts more effectively to produce pea varieties that best meet modern food production requirements.
The purpose of the research is to study biochemical parameters characterising seed quality in pea varieties of different morphotypes for the selection of varieties with improved nutritional properties.
Materials and methods
The object of research was the seeds of different morphotypes (leafless, leafy, heterophillous) varieties of peas (P. sativum) of Ukrainian and foreign breeding in the experiment of ecological variety testing of the Department of Breeding, Genetics and Seeding of Legume Crops of the Plant Breeding and Genetics Institute – National Center of Seed and Cultivar Investigation (PBGI – NCSCI): varieties of the leafless morphotype – ‘Kharkivskyi Etalonnyi’, ‘Mahnat’, ‘Metsenat’, ‘Oplot’, ‘Haiduk’, ‘Kamerton’, ‘Deviz’, ‘Chekryhinskyi’, ‘Tsarevych’ [originator Yuriev Plant Production Institute (Yuriev PPI), Ukraine], ‘Bilyi Anhel’, ‘Svit’, ‘Svit2’, ‘Darunok stepu’, ‘Hudevychi’ (originator PBGI – NCSCI, Ukraine), ‘Kombainovyi 1’, ‘Vusatyi 90’ [originator Luhansk National Agrarian University (LNAU), Ukraine], ‘Hotivskyi’ [originator of NSC “Institute of Agriculture of the National Academy of Agrarian Sciences of Ukraine” (NSC “IA NAAS”), Ukraine], ‘Achat’, ‘Zekon’, ‘Terno’ (originator Selgen, Czech Republic), ‘Astronavt’, ‘Madonna’, ‘Salamanka’ (originator “Norddeutsche Pflanzenzucht Hans-Georg Lembke KG”, Germany), ‘Profit’ (originator Limagrain, France), ‘Stabil’ (originator Saatbau Linz, Austria), ‘Petronium’ [originator Scientific and Production Agricultural Corporation Stepova Limited Liability Company (SPAC Stepova LLC), Ukraine], ‘Enduro’, ‘Balltrap’ (originator Florimond Desprez, France); leaf morphotype varieties – ‘Asket’, ‘Kharkivianyn’, ‘Intensyvnyi 92’ (originator Yuriev PPI, Ukraine), ‘Topaz’ (originator PBGI – NCSCI, Ukraine), ‘Luhanskyi’, ‘Blahodatnyi’ (originator LNAU, Ukraine], ‘Liulynetskyi Korotkosteblovyi’ (originator Uladovo-Liulynetska Research and Breeding Station of the Institute of Bioenergy Crops and Sugar Beet of the NAAS, Ukraine); varieties of the heterophillous morphotype (chameleon) – ‘Spartak’, ‘Orel’, ‘A31397’ (originator of ARRILC, Russia).
The field trials of ecological variety testing of peas were carried out in the selection crop rotation of the experimental base of the PBGI – NCSCI “Dachne”, located in the southern part of the Black Sea lowland (the terrain is represented by an almost ideal plain) in the steppe zone of the Odesa region, according to the generally accepted methods of field trials and technology of pea cultivation in 2021–2023. Sowing was carried out with the SKS-6-10 portioned seeder at a rate of 1.2 million germinating seeds per 1 ha. The plot size in the variety trial was 10 m2, replicated three times with a randomized arrangement of the varieties. Harvesting was carried out in a single stage at full grain maturity using a Sampo 130 combine harvester, followed by yield recording.
The soil cover is southern medium humus, highly loamy chernozems on loess deposits. The thickness of the humus layer is 40–50 cm and the humus content is 3.5–4.5%. The amount of absorbed bases is 40–45 mg-eq per 100 g of soil. Amount of available forms of nutrients (mg-eq per 100 g of soil) 3–4 nitrogen, 10–15 P2O5 and 20–30 K2O. The reaction of the soil solution is neutral or slightly alkaline (pH of the saline extract 6.0–7.2).
The climatic conditions in the study area are moderately warm, mainly under the influence of Atlantic and Mediterranean air masses. The average annual air temperature is +9.6 °C, and the sum of the effective temperatures is 3300 °C. Winters are mild and short. The coldest month is January, with a long-term average temperature of −2 °C. Spring arrives early, with temperatures exceeding +5 °C in the second or third decade of March. Summers are long and hot. The soil loses moisture in summer due to high temperatures and a drop in relative humidity of 35–40%, which leads to frequent dry winds. The temperature regime of the region does not limit the development of peas, but dry conditions are usually accompanied by higher temperatures, which inhibits plant growth. The aridity of the climate is caused not only by the lack of total rainfall (380–450 mm), but also by its uneven distribution during the growing season. Maximum summer rainfall usually occurs at the end of the pea growing season and often has no effect on plant growth and development. Inadequate rainfall is a factor limiting yields in the region. Spring and autumn are the driest seasons. The years of the trials were very different in terms of weather conditions – they were contrasting, with different levels of rainfall and heat. One of the important elements that characterize moisture availability is the hydrothermal coefficient (HTC): the ratio of moisture supply to moisture loss over a given period of time. Good moisture availability (HTC = 0.93–1.35) over the study period 2021–2023 was observed in our region in 2021, 2023, while near drought (HTC = 0.38) was observed in 2022, corresponding to the steppe zone.
Protein content was determined by the Kjeldahl method on a Kjltec Auto-1030 (FOSS) and protein fractionation was performed according to the method [6].
Protein electrophoresis was performed in 15% (PAGE) containing 1% sodium dodecyl sulphate (SDS) at pH 8.3 according to the Lemmley method [17] using a Hem-Hoff system (USA). The following protein mixture from Serva (Nimmechina) was used as molecular weight markers: phosphorylase B (97 kDa), bovine serum albumin (67 kDa), egg albumin (44 kDa), chymotrypsinogen (25 kDa), trypsin inhibitor (20.1 kDa), ribonuclease (13.7 kDa). Coomassie blue R-250 (Serva, Nijmegen) was used to visualize the electropherograms. The molecular weight of the components of the protein fractions was calculated according to the calibration curve of the relationship between log M and the relative mobility of the protein with respect to the mobility of bromophenol blue. The percentage of components in the electrophoretic spectra of the proteins was determined using Imagel image analysis software.
The amino acid composition of the proteins was analysed using a Hitachi-835 automatic amino acid analyzer (Japan). The proteins were hydrolyzed in 6 M hydrochloric acid at +105 °C in hermetically sealed bottles for 24 hours. After hydrolysis, the samples were evaporated in a rotary evaporator “Rotadest” (Hungary) and the precipitate was dissolved in 0.02 M hydrochloric acid.
Total flavonoid content was determined by a modified method [8]. Flavonoids were extracted with 70% ethanol at a 1 : 10 ratio of sample to extractant. The extraction was carried out in a water bath at +80 °C for 60 minutes. The method for the determination of flavonoids is based on the reaction of flavonoid complexation with aluminum chloride. The reaction of complexation with aluminum chloride develops within 40 minutes and the complex remains stable for 1 hour. The flavonoid whose maximum absorbance of the complex most closely matches the maximum absorbance of the complex with aluminum chloride of the sample under investigation (rutin) was used as a standard. The optical density of the resulting solution was measured on a spectrophotometer at a wavelength of 420 nm.
The activity of lipoxygenase was determined by the spectrophotometric method of coupled oxidation of β-carotene in the presence of linoleic acid at 440 nm. Lipoxygenase was extracted from ground pea seeds with petroleum ether (boiling point 40–60 °C). After removal of the ether, the material was mixed with 50 ml of water, shaken for one hour and centrifuged to obtain a clear solution. The extract obtained was treated with a small amount of active charcoal and filtered through a folded filter to remove the colour. The filtrate containing the lipoxygenase solution was stored in toluene at 5 °C under vacuum. The substrate used was linoleic acid obtained from fresh linseed oil by cold saponification with 10% KOH, followed by distillation of unsaturated fatty acids in a vacuum at +160 °C under 4 mm Hg and freezing at −20 °C. The resulting linoleic acid, with a refractive index of η20 = 1.4698, was stored in ampoules under vacuum at −5 °C. A solution of the sodium salt of linoleic acid was prepared immediately before the experiment by dissolving the calculated amount of linoleic acid in 0.1 n NaOH, based on a linoleic acid content of 1 mg per ml. The carotene solution contained 1.5 mg of crystalline carotene in 100 ml of a mixture consisting of 75 ml of twice-distilled acetone and 25 ml of alcohol. Lipoxygenase activity was inhibited with a 20% aqueous NaOH solution. To carry out the experiment, 47 ml of H2O and 3 ml of phosphate buffer at pH 6.5 were added to two 250 ml flasks, one of which was the control flask. Then 2 ml of 20% NaOH was added to the control flask. Then 1 ml of Na salt of linoleic acid, 5 ml of carotene solution and 0.1 to 1 ml of aqueous lipoxygenase extract were added to the flasks. After a fixed time, 2 ml of 20% NaOH was added to the test flask to stop the action of lipoxygenase.
Lipoxygenase activity was expressed in units of optical density at 440 nm per 1 mg per minute.
Trypsin inhibitor activity was determined by the decrease in the rate of casein hydrolysis in the presence of the inhibitor [18]. Lectin activity was determined by the Lutsik method [19].
The experiments were carried out in triplicate biological and analytical replicates. Statistical analysis of the results was performed using LibreOffice Calc software (GNU Lesser General Public License v3).
Results and discussion
The results of the research showed that the protein content in the seeds of pea varieties studied ranged from 20.5 to 25.7% on an absolutely dry basis: in varieties of the leafless morphotype, the average protein content was 22.7% ± 0.50; in varieties of the leafy morphotype – 23.1% ± 0.29; flavonoid content – from 43 to 67 μg/g of seeds: in the varieties of the leafless morphotype, the average flavonoid content was 60.2 μg/g ± 1.85; in the varieties of the leafy morphotype – 49.0 μg/g ± 3.46; trypsin inhibitor activity ranged from 1.35 to 1.65 g/kg of seeds: in varieties of the leafless morphotype, the average activity of trypsin inhibitors was 1.47 g/kg ± 0.04; in varieties of the leafy morphotype – 1.44 g/kg ± 0.007; lectin activity – from 0.002 to 0.003 μg/(mg protein)–1: in varieties of the leafless morphotype, the average lectin activity was 0.00276 μg/(mg protein)–1 ± 0.000133, in varieties of the leafy morphotype – 0.00239 μg/(mg protein)–1 ± 0.0006; lipoxygenase activity from 0.073 to 0.283 Δ234 U/mg protein: in the varieties of the leafless morphotype, the average lipoxygenase activity was 0.112 Δ234 U/mg protein ± 0.03, in the varieties of the leafy morphotype – 0.152 Δ234 U/mg protein ± 0.06. The varieties of the leafless morphotype included in the study were distinguished by a higher content of flavonoids compared to the varieties of the leaf morphotype (the difference between the mean values of the varieties is significant at P ≤ 0.05) (Table 1).
The proteins of the studied pea varieties are characterized by the presence of all the essential amino acids, including a high content of lysine, valine, phenylalanine, leucine and amino acids such as aspartic acid, glutamic acid, arginine, but are deficient mainly in the content of sulphur-containing amino acids (methionine and cysteine) (Table 2). Our amino acid analysis of pea seed proteins showed a low variability of this indicator in the varieties studied, probably due to the structure of their storage proteins [6, 7].
The study of the protein content in the seeds of a collection of pea varieties of different morphotypes showed a variation of this indicator from 18.99 to 26.36% on absolute dry basis: in varieties of the leafless morphotype the average protein content was 22.53% ± 0.29; in varieties of the leafy morphotype – 23.55% ± 0.34; in varieties of the heterophillous morphotype (chameleon) – 22.90% ± 0.7 (Table 3). The content of the main legume proteins – globulins, according to our data, depending on the pea variety, ranged from 55.4 to 79.0% of the total protein content. According to the literature [6], legumes (11S-globulin) and vicilin (7S-globulin) differ in the quantitative content of individual amino acids. According to our data, vicilin has less glutamic acid and aspartic acid, arginine, serine, lysine, while legume is enriched in these amino acids (Table 4), so it is very important to take into account the ratio of 11S/7S globulins.
The ratio of these fractions determines the functional properties of these proteins and thus the quality of the products and their technological properties.
The pea varieties studied differed in the quantitative content of vicilin and legumin and their ratio. Thus, the content of vicilin (7S globulin) varied from 8.33 to 22.50% of the total protein in pea seeds depending on the variety: the average vicilin content was 14.36% ± 0.53 in varieties of the leafless morphotype; 13.74% ± 0.7 in varieties of the leafy morphotype; 12.05% ± 2.4 in varieties of the heterophilous morphotype. The content of legumin (11S globulin) ranged from 13.60 to 37.34% of the total protein in seeds: in varieties of the leafless morphotype the average content of legumin was 22.34% ± 0.92; in varieties of the leafy morphotype – 25.24% ± 3.5; in varieties of the heterophillous morphotype – 17.15% ± 1.6. The ratio of 11S/7S globulins ranged from 0.95 to 2.53: in varieties of the mustachioed morphotype – 1.62 ± 0.1 (average for varieties); in varieties of the leafy morphotype – 1.84 ± 0.25; in varieties of the heterophillous morphotype – 1.50 ± 0.2 (Table 3).
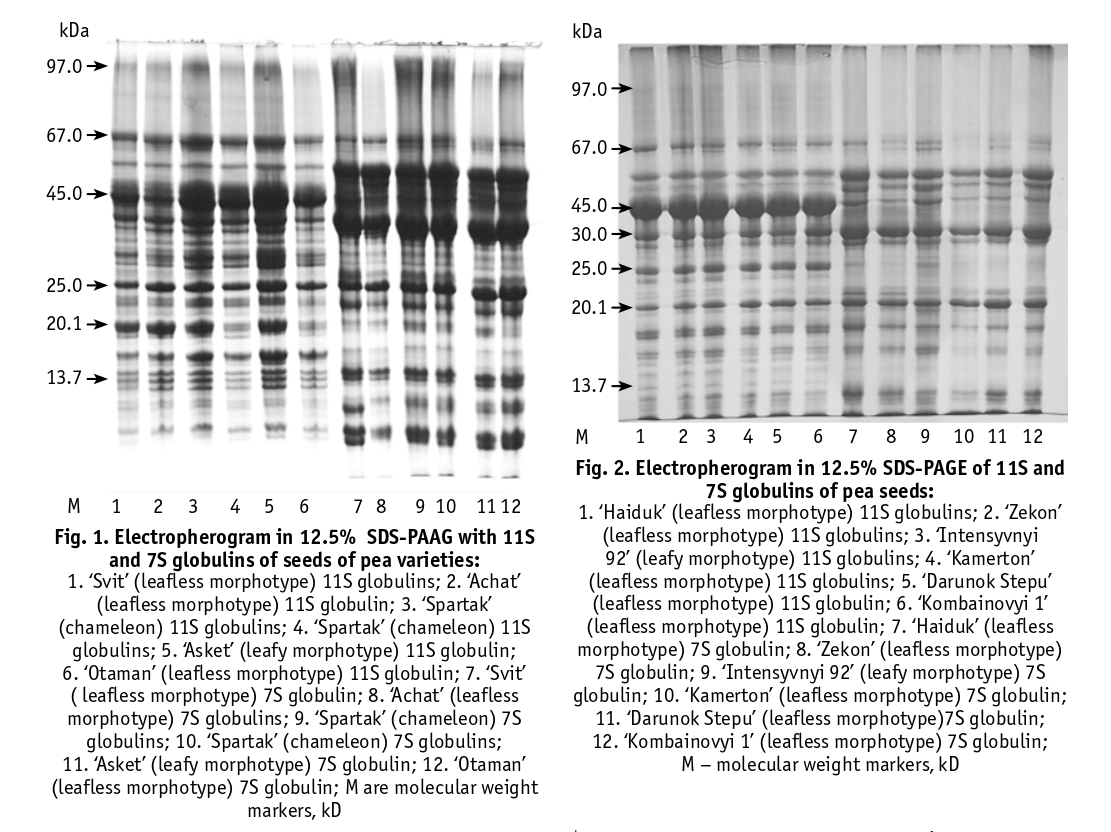
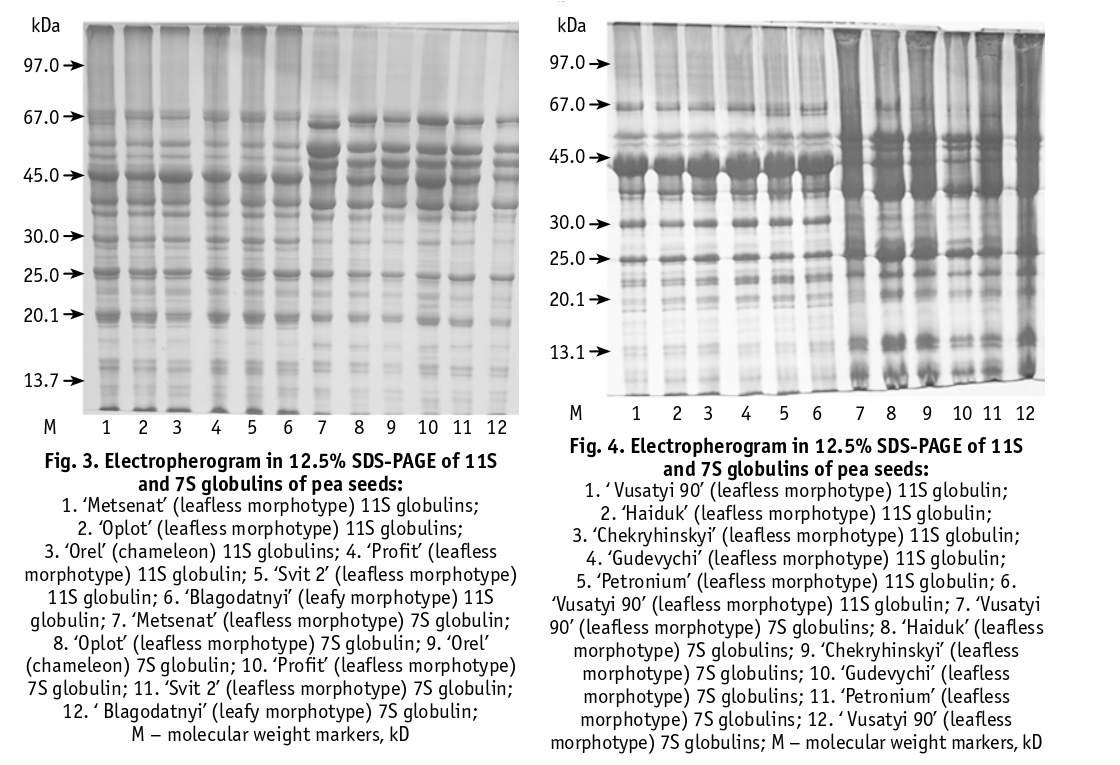
According to electrophoretic analysis (Figures 1–4), the 7S globulin protein fraction contained 29 polypeptide components and the 11S fraction contained 38 polypeptide bands with molecular weights ranging from 97 to 10 kDa. The 11S protein fraction contained the major components with molecular weights in the range of 60–67 kDa, 45 kDa, 40 kDa and 35 kDa, which according to the literature can be attributed to legumes [6, 20]. The component with a molecular weight of 40 kDa belongs to the acidic subunit of legumes. The electrophoretic spectrum of 7S globulins contains 5 components of vicilin with molecular weights in the range of 97 kDa, 65 kDa, 44–45 kDa and 35 kDa, 5 components of conglycinin with molecular weights in the range of 200–100 kDa, 63–67 kDa and 4 components of provicilin with molecular weights in the range of 66 kDa, 30 kDa, 20 kDa and 14.4 kDa. It has been shown that vicilin and convicilin, especially convicilin with a molecular weight of 63 kDa and vicilin with a molecular weight of 44 kDa, can cause an allergic reaction in the human body [21]. We found clear differences in the intensity of staining and displacement of globulin protein components of the same mobility level, as well as the presence and absence of components characteristic of a particular variety (Figures 1–4).
For example, in the electrophoretic spectrum of the 7S globulins in the ‘Kamerton’ variety, there are no components in the 67 and 25 kDa zone. In ‘Metsenat’, the intensity of the 7S-globulin components with a molecular weight of 66 and 50 kDa was significantly increased. In the variety ‘Intensyvnyi 92’ (leafy morphotype) the electrophoretic spectrum of 7S globulins contains components with a molecular weight of 25 and 14 kDa, which are absent in the varieties of the leafless morphotype ‘Zekon’, ‘Darunok Stepu’, ‘Kamerton’, ‘Kombainovyi 1’. In the electrophoretic spectrum of 11S globulins of the varieties ‘Petronium’, ‘Darunok Stepu’, ‘Metsenat’ a component with molecular weight 66 kDa was found, which is absent in other varieties of the leafless morphotype.

Having data on the quantitative content of total protein, 7S and 11S proteins and their ratio, component composition, flavonoid content and anti-nutrient compounds (trypsin inhibitors, lectins, lipoxygenase activity), it is possible to carry out specific studies on the selection of pea varieties for food use. For example, varieties with high levels of total protein, flavonoids and reduced content/activity of antinutrients are of great interest to breeders. Among the varieties studied, ‘Kharkivskyi Etalonnyi’ can be distinguished by these indicators. Differences in content, composition and structure of vicilin and legumin are manifested in both nutritional and functional properties. Legumin contains more sulphur-containing amino acids per unit of protein than vicilin, and their fraction is more nutritionally available. Vicilin has been shown to have better emulsifying properties than legumin. The ratio of these proteins has a significant effect on the protein extractability of peas. Varieties with higher levels of vicilin and/or lower levels of leguminous proteins have a higher protein extraction capacity than others [20]. It is very important to control the components of the globulins that can cause an allergic reaction in the human body, namely the component convicylin with a molecular weight of 63 kDa and vicilin with a molecular weight of 44 kDa, etc.
Among the varieties of the leafless morphotype studied, ‘Enduro’, ‘Balltrap’, ‘Tsarevych’ with high protein, legumin and 11S/7S globulin content and ‘Chekryhinskyi ‘ with high protein and vicillin content can be distinguished; among the varieties of the leaf morphotype, ‘ Intensyvnyi 92’, ‘Topaz’ with high protein, legume and 11S/7S globulin ratio and ‘Blahodatnyi’ with high vicilin content; among the varieties of the heterophilous morphotype (chameleon), ‘Spartak’ with high vicilin content and ‘Orel’ with high protein content (Table 3).
The structure and properties of pea proteins have been demonstrated to influence their structure-forming properties in food matrices such as emulsions, foams and gels. Current research indicates that each individual protein fraction possesses unique structure-forming properties, necessitating the implementation of specific fractionation processes to optimize these properties. In particular, the use of albumin, various globulins and mixed albumin-globulins, globulins has proven to be useful in specific food matrices, such as foams and emulsions [22]. This opens up prospects for the use of individual proteins in new food products and the determination of their content and ratio in pea seeds for the selection of food-grade varieties with specified technological parameters.
Conclusions
The study of biochemical parameters related to seed quality (protein content, flavonoids, lipoxygenase activity, trypsin inhibitor, lectins), the content of the main fractions of the protein complex (legumin and vicilin) and their ratio in seeds of different morphotypes showed the presence of varietal differences in the studied parameters. The electrophoretic and amino acid analyses revealed varietal differences in the relative content of individual protein components in the electropherogram, the presence / absence of some components in the electrophoretic spectra of vicilin and legumin, in their amino acid composition, which affect the nutritional value of pea seeds. The use of the biochemical criteria studied allows the selection of food-grade pea varieties with specific technological parameters.
References
Table 1
Biochemical characteristics of seeds of different pea varieties (2021–2022)
|
Variety |
Protein content, % |
Trypsin inhibitor activity, g/kg |
Lipoxygenase activity, ΔE234/mg |
Lectin activity, µg/(mg protein)–1 |
Flavonoid content, µg/g |
|
Varieties of the leafless morphotype |
|||||
|
‘Kharkivskyi Etalonnyi’ |
23.5 ± 0.14 |
1.57 ± 0.02 |
0.073 ± 0.004 |
0.002512 ± 0.0001 |
67.0 ± 0.34 |
|
‘Svit’ |
22.5 ± 0.12 |
1.45 ± 0.02 |
0.232 ± 0.002 |
0.00294 ± 0.0003 |
57.0 ± 0.22 |
|
‘Achat’ |
23.9 ± 0.11 |
1.55 ± 0.04 |
0.091 ± 0.001 |
0.00297 ± 0.0001 |
61.0 ± 0.31 |
|
‘Hotivskyi’ |
21.0 ± 0.089 |
1.35 ± 0.01 |
0.076 ± 0.002 |
0.00301 ± 0.0004 |
57.0 ± 0.28 |
|
‘Madonna’ |
22.6 ± 0.12 |
1.41 ± 0.02 |
0.086 ± 0.002 |
0.00237 ± 0.0001 |
59.0 ± 0.19 |
|
x |
22.7 ± 0.50 |
1.47 ± 0.04 |
0.112 ± 0.03 |
0.00276 ± 0.000133 |
60.2* ± 1.85 |
|
CV,% |
4.94 |
6.36 |
60.66 |
10.76 |
6.89 |
|
Varieties of leafy morphotype |
|||||
|
‘Intensyvnyi 92’ |
23.2 ± 0.11 |
1.32 ± 0.01 |
0.280 ± 0.002 |
0.00313 ± 0.0002 |
55.0 ± 0.21 |
|
‘Liulynetskyi Korotkosteblovyi’ |
22.6 ± 0.16 |
1.35 ± 0.09 |
0.080 ± 0.003 |
0.00106 ± 0.0001 |
43.0 ± 0.18 |
|
‘Topaz’ |
23.6 ± 0.091 |
1.65 ± 0.03 |
0.096 ± 0.004 |
0.00299 ± 0.0002 |
49.0 ± 0.19 |
|
x |
23.1 ± 0.29 |
1.44 ± 0.07 |
0.152 ± 0.06 |
0.00239 ± 0.0006 |
49.0* ± 3.46 |
|
CV,% |
2.18 |
12.67 |
73.12 |
48.34 |
12.24 |
* the difference between the mean values of the parameters of varieties of different morphotypes is significant at P = 0.05.
Table 2
Amino acid composition of pea seeds, mg/100 g of seeds
|
Amino acid |
Variety |
||
|
leafless morphotype |
leafy morphotype |
||
|
‘Svit’ |
‘Kharkivskyi’ |
‘Topaz’ |
|
|
Tryptophan |
0.17* ± 0.01 |
0.16* ± 0.02 |
0.18* ± 0.01 |
|
Lysine |
1.62* ± 0.03 |
1.47* ± 0.04 |
1.61* ± 0.02 |
|
Histidine |
0.40* ± 0.01 |
0.38* ± 0.01 |
0.40* ± 0.02 |
|
Arginine |
1.62* ± 0.05 |
1.42* ± 0.03 |
1.82* ± 0.03 |
|
Aspartic acid |
2.78* ± 0.03 |
2.43* ± 0.04 |
2.69* ± 0.04 |
|
Threonine |
0.84 ± 0.02 |
0.75 ± 0.01 |
0.84 ± 0.02 |
|
Serine |
0.95 ± 0.02 |
0.87 ± 0.01 |
0.94 ± 0.02 |
|
Glutamic acid |
3.54* ± 0.06 |
3.10* ± 0.05 |
3.39* ± 0.04 |
|
Proline |
0.93 ± 0.01 |
0.89 ± 0.01 |
0.93 ± 0.02 |
|
Glycine |
0.95 ± 0.01 |
0.79 ± 0.01 |
0.95 ± 0.02 |
|
Alanine |
0.92 ± 0.02 |
0.83 ± 0.01 |
0.92 ± 0.02 |
|
Valine |
1.09 ± 0.03 |
1.04 ± 0.04 |
1.07 ± 0.03 |
|
Methionine |
0.33* ± 0.01 |
0.24* ± 0.01 |
0.43* ± 0.02 |
|
Cysteine |
traces |
traces |
traces |
|
Isoleucine |
0.92 ± 0.01 |
0.86 ± 0.02 |
0.92 ± 0.02 |
|
Leucine |
1.56* ± 0.04 |
1.43* ± 0.03 |
1.56* ± 0.04 |
|
Tyrosine |
0.83 ± 0.02 |
0.72 ± 0.01 |
0.67 ± 0.01 |
|
Phenylalanine |
1.09 ± 0.05 |
0.99 ± 0.03 |
1.19 ± 0.04 |
* the difference between the content of the marked amino acids and the content of the other amino acids of each variety is significant at P ≤ 0.05.
Table 3
Protein, 11S and 7S globulins content and their ratio in seeds of pea varieties of different morphotypes (2022–2023)
|
Variety |
Protein content, % on abs. dry substance |
1S globulin (legumin) content, % of protein |
7S globulin (vicillin) content, % of protein |
11S/7S |
|
Varieties of the leafless morphotype |
||||
|
‘Achat’ |
21.00 |
15.76 |
13.26 |
1.19 |
|
‘Astronavt’ |
21.82 |
15.30 |
16.00 |
0.95 |
|
‘Mahnat’ |
22.21 |
17.71 |
14.68 |
1.21 |
|
‘Madonna’ |
23.27 |
19.74 |
12.61 |
1.56 |
|
‘Metsenat’ |
21.56 |
19.68 |
13.80 |
1.54 |
|
‘Oplot’ |
21.34 |
24.55 |
16.31 |
1.60 |
|
‘Profit’ |
20.49 |
26.40* |
13.71 |
2.04* |
|
‘Svit2’ |
23.06 |
18.37 |
13.01 |
1.57 |
|
‘Haiduk’ |
22.23 |
23.38 |
11.46 |
2.18 |
|
‘Zekon’ |
22.74 |
26.03* |
14.67 |
1.92 |
|
‘Kamerton’ |
22.05 |
24.62 |
12.68 |
2.09* |
|
‘Darunok Stepu’ |
22.84 |
22.87 |
12.54 |
1.99 |
|
‘Kombainovyi 1’ |
23.91 |
22.38 |
12.12 |
2.01* |
|
‘Stabil’ |
22.41 |
25.83* |
22.55* |
1.15 |
|
‘Terno’ |
21.88 |
23.62 |
12.42 |
1.90 |
|
‘Deviz’ |
18.99 |
13.60 |
12.77 |
1.06 |
|
‘Vusatyi 90’ |
21.98 |
18.09 |
17.87* |
1.01 |
|
‘Chekryhinskyi’ |
24.98* |
20.02 |
17.92* |
1.12 |
|
‘Hudevychi’ |
22.98 |
19.91 |
17.65* |
1.13 |
|
‘Petronium’ |
26.35* |
18.70 |
16.10 |
1.16 |
|
‘Ienduro’ |
22.77 |
29.50* |
11.21 |
2.63* |
|
‘Balltrap’ |
23.42 |
27.74* |
14.24 |
2.03* |
|
‘Tsarevych’ |
24.17* |
29.48* |
10.59 |
2.78* |
|
‘Salamanka’ |
22.48 |
29.23* |
14.73 |
1.98 |
|
‘Bilyi Anhel’ |
22.28 |
26.04* |
14.08 |
1.85 |
|
Average by morphotype |
22.53 ± 0.29 |
22.34 ± 0.92 |
14.36 ± 0.53 |
1.66 ± 0.10 |
|
Varieties of the leafy morphotype |
||||
|
‘Luhanskyi’ |
23.06 |
16.73 |
12.37 |
1.35 |
|
‘Blahodatnyi’ |
22.84 |
22.28 |
15.57 |
1.43 |
|
‘Intensyvnyi 92’ |
24.78* |
28.32* |
11.90 |
2.38* |
|
‘Topaz’ |
23.38 |
37.34* |
14.73 |
2.53* |
|
‘Kharkivianyn’ |
23.71 |
21.52 |
14.11 |
1.52 |
|
Average by morphotype |
23.55 ± 0.34 |
25.24 ± 3.54 |
13.74 ± 0.70 |
1.84 ± 0.25 |
|
Varieties of heterophillous morphotype (chameleon) |
||||
|
‘Spartak’ |
22.79 |
18.06 |
16.65* |
1.08 |
|
‘Orel’ |
24.26* |
19.36 |
11.16 |
1.73 |
|
‘А31397’ |
21.67 |
14.03 |
8.33 |
1.68 |
|
Average by morphotype |
22.90 ± 0.7 |
17.15 ± 1.6 |
12.05 ± 2.4 |
1.50 ± 0.20 |
|
x |
22.72 ± 0.24 |
22.31 ± 0.92 |
14.05 ± 0.47 |
1.68 ± 0.08 |
|
Max |
26.36 |
37.34 |
22.55 |
2.78 |
|
Min |
18.99 |
13.60 |
8.33 |
0.95 |
|
CV,% |
6.05 |
23.77 |
19.23 |
26.24 |
* the difference between the average value of the biochemical indicator across varieties and the value of the indicator for a particular variety is significant at P ≤ 0.05.
Table 4
Amino acid composition of vicilin and pea legumin, mg/100 mg
|
Variety |
Tyrosine |
Arginine |
Histidine |
Lysine |
Glutamic acid |
Aspartic acid |
Serine |
Proline |
Alanine |
Leucine |
Isolecine |
|
Varieties of the leafless morphotype |
|||||||||||
|
Vicilin (7S) |
|||||||||||
|
‘Kharkivskyi Etalonnyi’ |
3.66±0.049 |
1.55±0.023 |
0.69±0.037 |
3.43±0.051 |
9.13±0.017 |
7.55±0.081 |
2.66±0.013 |
9.44±0.061 |
2.46±0.034 |
7.40±0.06 |
4.60±0.035 |
|
‘Svit’ |
4.82±0.091 |
6.12±0.042 |
1.05±0.043 |
5.40±0.071 |
13.37±0.23 |
7.55±0.072 |
2.67±0.016 |
9.44±0.072 |
7.40±0.058 |
7.40±0.052 |
5.26±0.065 |
|
‘Achat’ |
1.11±0.037 |
0.40±0.017 |
1.56±0.026 |
1.11±0.009 |
1.05±0.017 |
0.78±0.024 |
0.32±0.011 |
0.62±0.015 |
0.40±0.009 |
1.26±0.012 |
1.06±0.005 |
|
x |
3.19±1.09 |
2.69*±1.75 |
1.10±0.25 |
3.31*±1.24 |
7.85*±3.61 |
5.29*±2.26 |
1.88*±0.78 |
6.50±2.94 |
3.42±2.07 |
5.35±2.04 |
3.64±1.30 |
|
Legumin (11S) |
|||||||||||
|
‘Kharkivskyi Etalonnyi’ |
1.82±0.010 |
6.43±0.069 |
1.56±0.031 |
4.43±0.039 |
1.42±0.014 |
8.52±0.089 |
3.24±0.02 |
5.17 ± 0.038 |
3.71±0.026 |
8.44±0.068 |
4.67±0.032 |
|
‘Svit’ |
3.63±0.059 |
3.68±0.028 |
1.44±0.012 |
4.92±0.051 |
14.85±0.143 |
3.14±0.032 |
3.46±0.06 |
6.13 ± 0.044 |
9.13±0.079 |
8.33±0.052 |
3.96±0.028 |
|
‘Achat’ |
4.23±0.080 |
7.13±0.085 |
1.44±0.011 |
5.61±0.062 |
20.52±0.162 |
10.31±0.11 |
4.10±0.08 |
2.99 ± 0.012 |
2.95±0.041 |
9.13±0.092 |
4.18±0.032 |
|
x |
3.22**±0.72 |
5.74*±1.05 |
1.48±0.04 |
4.98*±0.34 |
12.26*±5.66 |
7.32*±2.15 |
3.60*±0.26 |
4.76 ± 0.93 |
5.26**±1.94 |
8.63*±0.25 |
4.27±0.21 |
|
Varieties of the leafy morphotype |
|||||||||||
|
Vicilin (7S) |
|||||||||||
|
‘Topaz’ |
3.61*±0.032 |
2.49*±0.034 |
0.84±0.045 |
3.73*±0.045 |
6.40*±0.021 |
5.56*±0.035 |
2.24±0.023 |
4.46±0.042 |
2.74±0.028 |
7.3**±0.07 |
7.3**±0.049 |
|
Legumin (11S) |
|||||||||||
|
‘Topaz’ |
1.77*±0.027 |
6.00*±0.054 |
1.27±0.031 |
6.70**±0.078 |
17.82**±0.242 |
10.31**±0.112 |
3.65±0.03 |
5.60±0.042 |
2.83±0.034 |
9.63*±0.102 |
4.22±0.43 |
* the between the average content of the amino acid vicilin and leguminous by variety is significant at P ≤ 0.05;
** the difference between varieties of leafless and leafy morphotypes in the content of leguminous and vicilin amino acids is significant at P ≤ 0.05.
УДК 577.1
Молодченкова О. О.*, Коблай С. В., Тихонов П. С., Безкровна Л. Я., Рищакова О. В., Левицький Ю. А., Унтілова I. А. Біохімічний склад насіння різних сортів гороху. Plant Varieties Studying and Protection. 2024. Т. 20, № 2. С. 00–00. https://doi.org/10.21498/25181017.20.1.2024.304094
Селекційногенетичний інститут – Національний центр насіннєзнавства та сортовивчення, Овідіопольська дорога, 3, м. Одеса, 65036, Україна, *email: olgamolod@ukr.net
Мета. Вивчити біохімічні показники, що характеризують якість насіння, у різних за морфотипом сортів гороху для добору сортів з поліпшеними харчовими властивостями. Методи. Об’єктом досліджень було насіння 37 різних за морфотипом [листочковий, вусатий, гетерофільний (хамелеон)] сортів гороху вітчизняної та іноземної селекції. Використовували стандартні та розроблені в лабораторії методики біохімічного аналізу рослин (метод K’єльдаля, спектрофотометричні методи, електрофорез). Статистичний аналіз результатів досліджень здійснювали за допомогою програми «LibreOffice Calc» (GNU Lesser General Public License v3) та програми аналізу зображень «Imagel». Результати. Встановлено наявність сортових відмінностей за дослідженими біохімічними показниками, пов’язаними з якістю насіння (вміст білка, флавоноїдів, активність ліпоксигенази, інгібітора трипсину, лектинів), за вмістом основних фракцій білкового комплексу (легуміну та віциліну) та їхнім співвідношенням у насінні різних за морфотипом сортів. Завдяки електрофоретичному та амінокислотному аналізам виявлено сортові відмінності (у відносному вмісті окремих білкових компонентів на електрофореграмі, наявності / відсутності деяких компонентів в електрофоретичних спектрах віциліну та легуміну, їхньому амінокислотному складі), що впливають на харчову цінність насіння гороху. Висновки. Застосування досліджених біохімічних критеріїв дає змогу проводити добір сортів гороху продовольчого напряму із заданими технологічними параметрами.
Ключові слова: горох; якість насіння; білок; віцилін; легумін; флавоноїди; антипоживні фактори.
Надійшла / Received 02.04.2024
Погоджено до друку / Accepted 12.06.2024